REACTION RATES: KINETICS - PowerPoint PPT Presentation
REACTION RATES: KINETICS
particles move faster and collide with greater intensity ... examples: pulverizing, crushing, chopping up, etc. 3. agitation: increases reaction rate ... – PowerPoint PPT presentation
Title: REACTION RATES: KINETICS
1
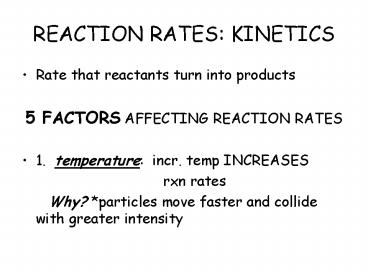
REACTION RATES KINETICS
- Rate that reactants turn into products
- 5 FACTORS AFFECTING REACTION RATES
- 1. temperature incr. temp INCREASES
- rxn rates
- Why? particles move faster and collide
with greater intensity
2
- 2. surface area increasing will cause reaction
rate to increase - Why? greater exposure of reacting
particles to each other - examples pulverizing, crushing, chopping
up, etc. - 3. agitation increases reaction rate
- Why? increases likelihood ofparticles being
exposed to one another increase in collisions - examples shaking, stirring
3
- 4. concentration increasing concentration of
reactants increases rate - Why? increasing particles increases
chance of collision resulting in a reaction - 5. catalysts substance that increases the
reaction rate without being used up in the
reaction. - Why? lowers the ACTIVATION energy
- sort of invites reacting particles together
so they will react
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.