Cycling of Matter PowerPoint PPT Presentation
Title: Cycling of Matter
1
Cycling of Matter
2
Recycling in the Biosphere
- In most organisms 95 of the body is made up of
just four elements. - Oxygen
- Carbon
- Hydrogen
- Nitrogen
3
The Cycle of Matter
- Matter changes form, but it does not disappear.
It can be used over and over again in a
continuous cycle.
4
The Water Cycle
5
The Water Cycle
- All living things need water to survive.
- Water moves between the ocean, atmosphere, and
land.
6
The Water Cycle
- Water molecules enter the atmosphere as water
vapor (a gas), when they evaporate from the ocean
or other bodies of water. - Evaporation
- The process by which water changes from liquid
form to an atmospheric gas.
7
The Water Cycle
- Transpiration
- Water can enter the atmosphere by evaporating
from the leaves of plants.
8
The Water Cycle
- Sun heats the atmosphere.
- Warm, moist air rises, and cools.
- Water vapor condenses into droplets that form
clouds. - As droplets become large enough, they return to
the Earths surface in the form of precipitation.
9
The Water Cycle
- Most precipitation runs along the surface of the
earth until it enters a river or stream that
carries the runoff back to a lake or ocean. - Rain also seeps into the soil, some deeply enough
to become ground water.
10
The Water Cycle
11
Nutrient Cycles
12
Nutrient Cycles
- Nutrients
- All the chemical substances that an organism
needs to sustain life. - Every living organism needs nutrients to build
tissues and carry out essential life functions. - Nutrients are passed between organisms and the
environment through biogeochemical cycles.
13
Elements essential for life also cycle through
ecosystems.
- Biogeochemical cycles
- A biogeochemical cycle is the movement of a
particular chemical through the biological and
geological, or living and non-living, parts of an
ecosystem. - Many substances will change states, from solid,
to liquid, to gas, as they move through their
cycles.
14
The Oxygen Cycle
- Plant, animals, and most other organisms need
oxygen for cellular respiration.
15
The Oxygen Cycle
16
The Carbon Cycle
- Carbon is a key ingredient of living tissue.
- Calcium carbonate (CaCO3) is an important
component of animal skeletons, and is found in
several kinds of rocks. - Carbon dioxide (CO2) is an important part of the
atmosphere. - CO2 is taken up by plants during photosynthesis,
and given off by both plants and animals during
cellular respiration.
17
The Carbon Cycle
- Biological processes, such as photosynthesis,
respiration, and decomposition, take up and
release carbon and oxygen.
18
The Carbon Cycle
- Geochemical processes, such as erosion and
volcanic activity, release carbon dioxide to the
atmosphere and oceans.
19
The Carbon Cycle
- Mixed biogeochemical processes, such as the
burial and decomposition of dead organisms and
their conversion under pressure into coal and
petroleum (fossil fuels), store carbon
underground.
20
The Carbon Cycle
- Human activities, such as mining, cutting and
burning forests, and burning fossil fuels,
release carbon dioxide into the atmosphere.
21
The Carbon Cycle
- Plants take in CO2 and use carbon to build
carbohydrates during photosynthesis. - The carbohydrates are passed along food webs to
animals and other consumers.
22
The Carbon Cycle
- In the ocean, carbon is found along with calcium
and oxygen in the form of calcium carbonate. - Calcium carbonate is formed by marine organisms.
- This accumulates in marine sediments and in the
bones and shells of organisms.
23
The Carbon Cycle
- Not all carbon molecules move freely thorough the
cycle. - Areas that store carbon over a long period of
time are called carbon sinks. - Example Forest land, where large amounts of
carbon are stored in the cellulose of wood.
24
The Carbon Cycle
25
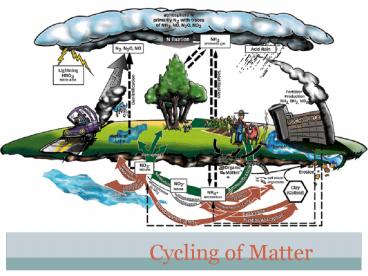
The Nitrogen Cycle
- All organisms require nitrogen to make amino
acids, which in turn are used to make proteins. - Many different forms of nitrogen are found in the
biosphere.
26
The Nitrogen Cycle
- Nitrogen gas (N2) makes up 78 of Earths
atmosphere.
27
The Nitrogen Cycle
- Ammonia (NH3), nitrate (NO3-), nitrite (NO2-),
are found in wastes produced by many organisms
and in dead and decaying organic matter.
28
The Nitrogen Cycle
- Nitrogen exists in several forms in the ocean and
other large bodies of water.
29
The Nitrogen Cycle
- Human activity adds nitrogen to the biosphere in
the form of nitratea major component of plant
fertilizers.
30
The Nitrogen Cycle
- Although nitrogen gas is the most abundant form
of nitrogen on Earth, only certain types of
bacteria can use this form directly. - These bacteria live in the soil and on the roots
of legumes, convert nitrogen gas into ammonia. - The process is called NITROGEN FIXATION.
31
The Nitrogen Cycle
- Now, other bacteria can convert the ammonia into
nitrates and nitrites. - Producers (plants) can use nitrates and nitrites
to make proteins.
32
The Nitrogen Cycle
- Consumers then eat the producers and reuse the
nitrogen to make their own proteins.
33
The Nitrogen Cycle
- When the consumers die, decomposers return
nitrogen to the soil as ammonia. - This process is called DENITRIFICATION.
34
The Nitrogen Cycle
35
The Nitrogen Cycle
36
The Phosphorus Cycle
- Phosphorous is essential to living organisms
because it forms part of important
life-sustaining molecules such as DNA and RNA. - Phosphorous is not very common in the biosphere.
37
The Phosphorous Cycle
- Phosphorous is found mainly on land in rocks and
soil minerals, and in ocean sediments. - As the rocks and sediments wear down, phosphate
is released.
38
The Phosphorous Cycle
- On land, some of the phosphate washes into rivers
and streams, where it dissolves. - The phosphate eventually makes its way to the
ocean, where it is used by marine organisms.
39
The Phosphate Cycle
- Some phosphate stays on land and cycles between
organisms and the soil. - When plants absorb phosphate from the soil or
from water, the plants bind the phosphate into
organic compounds.
40
The Phosphate Cycle
41
Nutrient Limitation
- Primary Productivity
- The rate at which organic matter is created by
producers. - One factor that controls primary productivity of
an ecosystem is the amount of available
nutrients. - If a nutrient is in short supply, it will limit
an organisms growth.
42
Nutrient Limitation
- Limiting nutrient
- When an ecosystem is limited by a single nutrient
that is scarce or cycles very slowly. - These ecosystems are considered to be
nutrient-poor environments.
43
Nutrient Limitation
- Oceans can be considered to be nutrient-poor.
- Sea water contains only .00005 nitrogen
(1/10,000 of the amount typically found in soil).
- In sea water and other saltwater environments,
nitrogen is often the limiting nutrient. - In streams, lakes, and freshwater environments,
phosphorous is typically the limiting nutrient.
44
Nutrient Limitation
- Farmers are aware of nutrient limitation and
apply fertilizer to their crops. - Fertilizer contains nitrogen, phosphorous, and
potassium.
45
Nutrient Limitation
- As rain comes down on fertilized fields, runoff
will flow into the oceans and freshwater lakes
and ponds. - The results are ALGAL BLOOMS.