Cyclic voltammetry for LiCoO2 deposited on Fsi (Flat-Si) and ESi (Etched-Si) - PowerPoint PPT Presentation
Title:
Cyclic voltammetry for LiCoO2 deposited on Fsi (Flat-Si) and ESi (Etched-Si)
Description:
No differences in basic electrochemical characteristics between two ... Diffusion kinetics as a function of film thickness Electrochemical Impedance Spectroscopy ... – PowerPoint PPT presentation
Number of Views:342
Avg rating:3.0/5.0
Title: Cyclic voltammetry for LiCoO2 deposited on Fsi (Flat-Si) and ESi (Etched-Si)
1
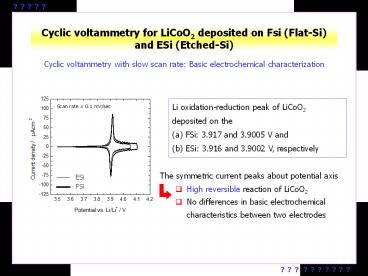
Cyclic voltammetry for LiCoO2 deposited on Fsi
(Flat-Si) and ESi (Etched-Si)
Cyclic voltammetry with slow scan rate Basic
electrochemical characterization
- Li oxidation-reduction peak of LiCoO2 deposited
on the - FSi 3.917 and 3.9005 V and
- ESi 3.916 and 3.9002 V, respectively
The symmetric current peaks about potential axis
- High reversible reaction of LiCoO2
- No differences in basic electrochemical
characteristics between two electrodes
2
Continued
Cyclic voltammetry with variation of scan-rate
Scan rate 0.1 2 mV/sec
- Higher anodic and lower cathodic peak potential
for ESi than FSi with increase in scan rates. - Larger ionic and electronic resistance for film
on ESi substrate than on FSi substrate
3
Rate-capability for LiCoO2 deposited on FSi and
ESi
Current density range 10 ?A/cm2 1 mA/cm2
4
SEM photos for LiCoO2 deposited on FSi and ESi
Deposition time for both films 8 hrs
5
Electrical resistance of current collector
Sample Length ? With (cm) Resistance (?)
Pt on the FSi substrate 2.5 ? 1 2.4
Pt on the ESi substrate 2.5 ? 1 4.1
Pt on the alumina substrate 2.5 ? 1 4.3
6
Cyclic voltammetry for LiCoO2 deposited on
alumina substrate
- No differences in basic electrochemical
characteristics between two electrodes - The largest peak potential divergence for alumina
substrate
7
Rate-capability for LiCoO2 deposited on FSi and
ESi
- Alumina
- At 1 mA/cm2, 80 capacity retention
- The worst rate-capability among three substrates
- Similar capacity at 10 ?A/cm2 to the FSi
8
SEM photos for LiCoO2 deposited on alumina
substrate
9
Cyclability of LiCoO2 deposited on the FSi and
alumina substrate
Current density 50 ?A/cm2
- Good cyclability of LiCoO2 deposited on both
substrate at low current density (50 ?A/cm2)
10
Rate-capability of LiCoO2 as a function of film
thickness
Charge-discharge variation 10 ?A/cm2 1
mA/cm2 Film-thickness variation 1500 6000 Å
- Diffusion length for Li ion ? film thickness
- Film thickness ? ? Rate-capability ? ???
however, - Film thickness ? ? Rate-capability ?
- !!! Diffusion kinetics as a function of film
thickness
11
Electrochemical Impedance Spectroscopy (EIS) for
LiCoO2 deposited on the Fsi substrate
12
Li-ion diffusion coefficient measured by EIS and
CV
- Similar trend by EIS at 3.9 V and by CV
- Diffusion coefficient increases with equilibrium
voltage and film thickness - Deintercalation of Li ? generates the
intercalation-induced stress
13
Stress measurement by optical cantilever method
- Negative sign on deflection angle compressive
stress - Increase in charge current density ? decrease in
deflection angle - ? Decrease in expansion depth by steep
concentration gradient
14
Calculated tress field assumed linear distribution
Stress induced by charge reaction
Stress field divided by film thickness
- Stress calculation by Stoney equation ?
- Amount of stress induced by charge reaction
- Increase with film thickness, however
- Decrease with film thickness for stress field
induced by charge reaction - Diffusion coefficient decrease with film
thickness
15
Charge-discharge properties for anode and
full-cell
Amorphous-Si anode
Full-cell
Current density 50 ?A/cm2 Thickness 350 Å
16
Operation of digital clock by all-solid-state Li
microbattery
- The first cell in the world using an amorphous-Si
anode - Back-up for about 7 hrs upon 1 charge
- Showing the possibility of practical utilization
of microbattery