Exp 19A: Oxidation-Reduction Reactions PowerPoint PPT Presentation
1 / 19
Title: Exp 19A: Oxidation-Reduction Reactions
1
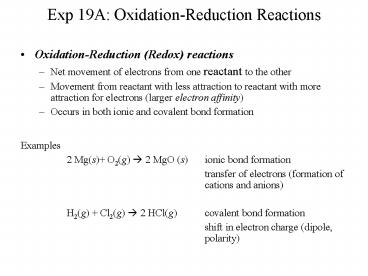
Exp 19A Oxidation-Reduction Reactions
- Oxidation-Reduction (Redox) reactions
- Net movement of electrons from one reactant to
the other - Movement from reactant with less attraction to
reactant with more attraction for electrons
(larger electron affinity) - Occurs in both ionic and covalent bond formation
- Examples
- 2 Mg(s) O2(g) ? 2 MgO (s) ionic bond formation
- transfer of electrons (formation of
cations and anions) - H2(g) Cl2(g) ? 2 HCl(g) covalent bond
formation - shift in electron charge (dipole,
polarity)
2
Exp 19A Oxidation-Reduction Reactions
Oxidation loss of electrons Mg ? Mg2
2e- Reduction gain in electrons ½ O2 2e-
? O2-
transfer or shift of electrons
X looses electron(s)
Y gains electron(s)
X is oxidized
Y is reduced
X is the reducing agent
Y is the oxidizing agent
X increases its oxidation number
Y decreases its oxidation number
- reducing agents loose electrons while oxidizing
agents gain electrons simultaneously - a chemical change cannot be an oxidation
reaction or just a reduction reaction - it is always an oxidation-reduction reaction
3
Exp 19A Oxidation-Reduction Reactions
- Goal of the Experiment (I)
- Observe redox reactions between halogens
(oxidizers) and halogen ions (halides, reducers)
in cyclohexane - Halide ions (polar!) are insoluble in non-polar
cyclohexane - Halogens (non-polar!) are soluble in cyclohexane
- If a reaction takes place, the color in the
cyclohexane may be different from the color in
water, but .. - You have to make very careful observations and
THINK about the results
4
Redox Activity of Halogens
- F2 gt Cl2 gt Br2 gt I2
- Redox potential and reactivity decreases down
Group 7A - A halogen higher in the periodic system oxidizes
one that is lower in the periodic system - Chlorine can oxidize bromide
- -1 0 0 -1
- 2 Br- (aq) Cl2 (aq) ? Br2 (aq) 2 Cl- (aq)
- Bromine cannot oxidize chloride
- 2 Cl- (aq) Br2 (aq) ? no reaction
5
Exp 19A Oxidation-Reduction Reactions
- Goal of the Experiment (II)
- Observe redox reactions with permanganate and
iron(III) ions - Reduction of
- MnO4- (permanganate, purple) to MnO42-
(manganate, green) - MnO4- (permanganate, purple) to MnO2 (manganese
oxide, black solid) - MnO4- (permanganate, purple) to Mn2
(manganese(II) ion, pink) - Fe3 (iron(III), reddish brown) to Fe2(iron(II),
pale green)
6
Exp 19A Oxidation-Reduction Reactions
- Cl2 Solution
- Reaction
- 2OCl- 4H3O 2e- ? Cl2 6H2O
- 2Cl- ? Cl2 2e-
- 2OCl- 2Cl- 4 H3O ? 2Cl2 6H2O
- or
- OCl- Cl- 2 H3O ? Cl2 3H2O
- Preparation of 12 mL 0.050 M chlorine water (add
in this order and in the fume hood and leave it
there!) - 1.70 mL bleach (NaOCl)
- 1.2 ml 0.50 M H2SO4
- 6.0 mL 0.20 M NaCl
- 3.1 mL H2O
- Put in large test tube
7
Exp 19A Oxidation-Reduction Reactions
- Br2 Solution
- Reaction
- 2BrO3- 12 H3O 10e- ? Br2 18H2O
- 10 Br- ? 5 Br2 10e-
- 2BrO3- 10 Br- 12 H3O ? 6 Br2 18H2O
- or
- BrO3- 5 Br- 6 H3O ? 3 Br2 9H2O
- Preparation of 12 mL 0.050 M bromine water
- 1.0 mL 0.2 M KBrO3
- 5.0 mL 0.20 M NaBr
- 1.2 ml 0.50 M H2SO4
- Wait 15 minutes before adding
- 4.8 mL H2O
- Put in large test tube
8
Exp 19A Oxidation-Reduction Reactions
- I2 Solution
- Reaction
- 2Cu2 2I- 2e- ? 2CuI
- 2I- ? I2 2e-
- 2Cu2 4I- ? 2CuI I2
- Preparation of 12 mL 0.050 M iodine water
- 6.0 mL 0.20 M CuSO4
- 6.0 mL 0.40 M NaI
- Filter over double filter paper to remove
insoluble CuI - Put solution in large test tube
9
Exp 19A Oxidation-Reduction Reactions
- Exp 1
- Put 15 drops of Cl2 solution in small test tube
- Put 15 drops of Br2 solution in 2nd small test
tube - Put 15 drops of I2 solution in 3rd small test
tube - Add 1 mL cyclohexane to each tube
- Shake or swirl solution gently
- Record colors (if any) in cyclohexane layer
- Keep these tubes as reference colors
10
Exp 19A Oxidation-Reduction Reactions
- Exp 2 Reactions of Halogens with Halides (I)
- Put 10 drops of 0.2 M NaCl in 2 clean and dry
test tubes - Add 10 drops of Br2 solution to tube 1
- Add 10 drops of I2 solution to tube 2
- Add 1 mL cyclohexane to each tube
- Shake or swirl
- Record color change for both the aqueous and the
organic layer - NO reaction cyclohexane will have color of
aqueous layer - Reaction color change in cyclohexane
11
Exp 19A Oxidation-Reduction Reactions
- Exp 2 Reactions of Halogens with Halides (II)
- Clean test tubes
- Put 10 drops of 0.2 M NaBr in 2 clean and dry
test tubes - Add 10 drops of Cl2 solution to tube 1
- Add 10 drops of I2 solution to tube 2
- Add 1 mL cyclohexane to each tube
- Shake or swirl
- Record color change for both the aqueous and the
organic layer
12
Exp 19A Oxidation-Reduction Reactions
- Exp 2 Reactions of Halogens with Halides (III)
- Clean test tubes
- Put 5 drops of 0.4 M NaI in 2 clean and dry test
tubes - Add 10 drops of Cl2 solution to tube 1
- Add 10 drops of Br2 solution to tube 2
- Add 1 mL cyclohexane to each tube
- Shake or swirl
- Record color change for both the aqueous and the
organic layer
13
Exp 19A Oxidation-Reduction Reactions
- Exp 3 Reactions of Permanganate and Iron (I)
- Clean test tubes
- Put 15 drops of 0.2 M NaBr in two test tubes
- Add 1 drop of 0.1 M KMnO4 solution to tube 1
- Add 5 drops of 0.1 M FeCl3 solution to tube 2
- Add 5 drops of 3 M H2SO4 to each tube
- Add 1 mL cyclohexane to each tube
- Shake or swirl
- Record color change/observations for both the
aqueous and the organic layer
14
Exp 19A Oxidation-Reduction Reactions
- Exp 3 Reactions of Permanganate and Iron (II)
- Clean test tubes
- Put 8 drops of 0.4 M NaI in two test tubes
- Add 1 drop of 0.1 M KMnO4 solution to tube 1
- Add 5 drops of 0.1 M FeCl3 solution to tube 2
- Add 5 drops of 3 M H2SO4 to each tube
- Add 1 mL cyclohexane to each tube
- Shake or swirl
- Record color change/observations for both the
aqueous and the organic layer
15
Exp 19A Oxidation-Reduction Reactions
- Exp 3 Reactions of Permanganate and Iron (III)
- Clean test tubes
- Put 8 drops of 0.4 M NaI in one test tube
- Add 5 drop of 6.0 M NaOH solution to tube
- Add 1 drop of 0.1 M KMnO4 solution to tube
- Add 1 mL cyclohexane to tube
- Shake or swirl
- Record color change/observations for both the
aqueous and the organic layer
16
Exp 19A Oxidation-Reduction Reactions
- Exp 3 Reactions of Halogens and Halides
- Cl- Br2 NR Br2 is still present
(yellow-orange color) - Cl- I2 NR I2 is still present (violet color)
- Cl2 2Br- ? 2Cl- Br2 (yellow-orange color)
- I2 2Br- NR I2 is still present (violet
color) - Cl2 2I- ? 2Cl- I2 (violet color)
- Br2 2I- ? 2Br- I2 (violet color)
17
Exp 19A Oxidation-Reduction Reactions
- Exp 3 Reactions of Permanganate and Iron
- (7) 2MnO4- 16H3O 10e- ? 2Mn2 8H2O
- 10Br- ? 5Br2 10e-
- 2MnO4- 10Br- 16H3O ? 2Mn2 5Br2 8H2O
(yellow-orange color) - (9) 2MnO4- 16H3O 10e- ? 2Mn2 8H2O
- 10I- ? 5I2 10e-
- 2MnO4- 10I- 16H3O ? 2Mn2 5I2 8H2O
(violet color) - (8) Fe3 Br- ? No color, No Reaction
- (10) 2Fe3 2 e- ? 2Fe2
- 2I- ? I2 2e-
- 2Fe3 2I- ? 2Fe2 I2 (violet color)
18
Exp 19A Oxidation-Reduction Reactions
- Exp 3 Reactions of Permanganate and Iron
- (11) 6MnO4- 6e- ? 6MnO42-
- I- 3H2O ? IO3- 6H3O 6e-
- 6H3O 6OH- ? 6H2O
- 6MnO4- I- 6OH- ? 6MnO42- IO3- 3H2O
- green color in aqueous layer
- no color in cyclohexane
- no formation of a nonpolar halogen
19
Exp 19A Oxidation-Reduction Reactions
- Post Lab
- Results sheets (p. 351-352)
- Post lab questions 1, 2a-d, 3a-c
- Give balanced equations for every reaction that
happened - If there was no reaction, write NR and indicate
how you reached that conclusion - Answer the questions