REVERSIBLE REACTIONS PowerPoint PPT Presentation
1 / 39
Title: REVERSIBLE REACTIONS
1
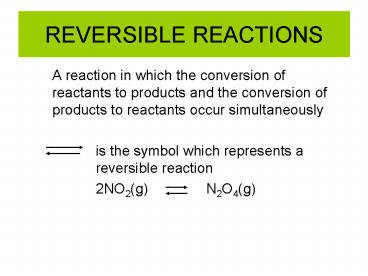
REVERSIBLE REACTIONS
- A reaction in which the conversion of reactants
to products and the conversion of products to
reactants occur simultaneously - is the symbol which represents a reversible
reaction - 2NO2(g) N2O4(g)
2
CHEMICAL EQUILIBRIUM
- Chemical equilibrium happens in a reversible
reaction, when the rate at which a substance
reacts is exactly the same as the rate at which
it is being produced. - 2NO2(g) N2O4(g)
- In equilibrium the concentrations of NO2 N2O4
would not change.
3
LE CHATELIERS PRINCIPLE
- When a change happens to a reaction at
equilibrium, the reaction will shift to reduce
the effects of that change. - Concentration
- If more of a substance is added, the
concentration of that substance increases. More
of that substance will be used and the reaction
will shift towards the other side of the
equation. - If substance is removed, the concentration of
that substance decreases, and the reaction will
shift to create more of that substance.
4
(No Transcript)
5
LE CHATELIERS PRINCIPLE
- Consider the following chemical reaction at
- equilibrium
- H2CO3(aq) ? CO2(aq) H2O(l)
- What will adding more CO2 do to the equilibrium?
- What will adding more H2CO3 do to the
equilibrium?
6
LE CHATELIERS PRINCIPLE
- When a change happens to a reaction at
equilibrium, the reaction will shift to reduce
the effects of that change. - Temperature (kJ in an equation)
- If temperature is added to a system the reaction
will shift away from the side of the reaction
that requires heat. - If the temperature of a system decreases, the
reaction will shift towards the side of the
reaction that requires heat. - 50 kJ NH4Cl(s) NH3(g) HCl(g)
7
LE CHATELIERS PRINCIPLE
- ExampleConsider the following chemical reaction
at equilibrium - 2SO2(g) O2(g) ? 2SO3(g) 22 kJ
- What effect will heating this reaction have on
its equilibrium? - b) What effect will cooling this reaction have
on its equilibrium?
8
LE CHATELIERS PRINCIPLE
- When a change happens to a reaction at
equilibrium, the reaction will shift to reduce
the effects of that change. - Pressure (comes from the moles of gas)
- If the total pressure of a system is increased,
the system will shift to reduce that pressure by
proceeding in the direction that produces fewer
molecules of gas.
9
LE CHATELIERS PRINCIPLE
Consider the following chemical reaction at
equilibrium 2SO2(g) O2(g) ? 2SO3(g)
heat What effect will adding pressure to this
reaction have on its equilibrium? What effect
will decreasing the pressure of this reaction
have on its equilibrium?
10
LE CHATELIERS PRINCIPLE
Consider the following chemical reaction at
equilibrium 2SO2(g) O2(g) ? 2SO3(g)
heat What effect will adding pressure to this
reaction have on its equilibrium? What effect
will decreasing the pressure of this reaction
have on its equilibrium?
11
LE CHATELIERS PRINCIPLE
Consider the following reaction at
equilibrium PCl5(g) heat ? PCl3(g)
Cl2(g) What could be done to this system to
increase the amount of PCl3 produced? What
could be done to this system to increase the
amount of PCl5 produced?
12
EQUILIBRIUM CONSTANT
- The ratio of product concentrations to reactant
- concentrations at equilibrium
- Shows Whether the reactants (Keqlt1) or
products (Keq gt1) are favored in a reaction - aA bB ? cC dD
- Keq Cc?Dd
- Aa?Bb
The reversible reaction N2(g) 3H2(g) ?
2NH3(g) produces ammonia, which is a fertilizer.
At equilibrium, a 1 L flask contains 0.15 mol
H2, 0.25 mol N2, and 0.10 mol NH3. Calculate the
Keq for the reaction.
13
Solubility Product Constant
- Same as the equilibrium constants but only shows
the ion concentrations (as that is what is
dissolved in the solution) - Shows how soluble the compound is
- The lower the solubility product constant, the
lower the solubility of the compound
14
- pH lt 7
- Sour/tart taste
- Electrolytes in solution
- React with many metals to produce hydrogen gas
- React with bases to form water and salt
- Will change the color of an indicator
- Acidic solutions can be formed when nonmetal
oxides react with water
ACIDS
15
- pH gt 7
- Bitter taste
- Slippery feel
- Electrolytes as aqueous solutions
- Will cause an indicator to change color
- React with acids to form water and salt
- Basic solutions can be formed when a metal oxide
reacts with water
Bases
16
DEFINITIONS OF ACIDS/BASES
ACID BASE
Arrhenius Hydrogen-containing compounds that ionize to yield hydrogen ions (H) in aqueous solution Compounds that ionize to yield hydroxide ions (OH-) in aqueous solution
Bronsted-Lowry Hydrogen-ion donor Hydrogen-ion acceptor
17
DEFINITIONS OF ACIDS/BASES
ACID BASE
Arrhenius Start with H (hydrogen ion) Hydrogen-containing compounds that ionize to yield hydrogen ions (H) in aqueous solution End with OH (hydroxide ion) Compounds that ionize to yield hydroxide ions (OH-) in aqueous solution
18
ACIDS
19
(No Transcript)
20
CONJUGATE ACID/BASE PAIRS
- Consists of two substances related by the loss or
gain of a single hydrogen ion (H) - Conjugate acid Particle formed when a base
gains a hydrogen ion (H) - Hydronium Ion (H3O) A water molecule that
gains a hydrogen ion and becomes positively
charged - Conjugate base The particle that remains when
an acid has donated a hydrogen ion
21
Acid (donates H) Conjugate Base
HCl
HNO3
Base (accept H) Conjugate Acid
OH-
NH3
22
CONJUGATE ACID/BASE PAIRS
- Identify the acid, base, conjugate acid and
- conjugate base in each equation.
- HCl NH3 ? NH4 Cl-
- HCO3- HCl ? H2CO3 Cl-
- HCO3-1 OH- ? H2O CO32-
23
ACID (donates a H) BASE (Accepts H) Conjugate Acid (Accepted H) Conjugate Base
HClNH3 ? NH4 Cl- HClNH3 ? NH4 Cl- HClNH3 ? NH4 Cl- HClNH3 ? NH4 Cl-
NH4 H2O
HPO4-2 NO3-
24
Which number is larger?
- 1.0 x 10-7 or 1.0 x 10-5
- 2.8 x 10-11 or 1.0 x 10-7
- 3.2 x 10-2 or 1.0 x 10-7
- 1.0 X 10-7 or 3.7 x 10-13
25
(No Transcript)
26
Neutral Solutions
OH- H if the solution is neutral
pH of H2O 7
If H is greater than OH- than the solution
is acidic If OH- is greater than H than the
solution is basic
27
Solutions
If H increases than OH- must decrease If
OH- increases than H must decrease
28
Mathematical formula to link the concentration of
H to OH- 1 x 10-14 H x OH- If the
solution is neutral than H OH- H
1.0 x 10-7 OH- 1.0 x 10-7 If the solution
is acidic than H is larger than 1.0 x 10-7 If
the solution is basic than OH- is larger than
1.0 x 10-7
29
1 x 10-14 H x OH-
- If the H in a solution is 1.0 x 10-5 M, what
is the OH- of this solution? Is this solution
acidic, basic or neutral?
- 2) If the hydroxide ion concentration of an
aqueous solution is 1 x 10-3 M, what is the H
in the solution? Is this solution acidic, basic
or neutral?
30
Self-Ionization of Water
- Happens when the collisions between water
molecules are - energetic enough to transfer a hydrogen ion from
one water - molecule to another.
- Hydrogen ions in solution are called
- Protons Hydrogen Ions (H) Hydronium Ions
(H3O)
31
Neutral Solutions
- Water is neutral so a neutral solution would have
equal concentrations of H and OH- - In pure water at equilibrium, the product of the
hydrogen-ion concentration and the hydroxide ion
concentration equals 1.0 x 10-14 - H x OH- 1.0 x 10-14
- So in a neutral solution
- H 1.0 x 10-7
- OH- 1.0 x 10-7
32
- In equilibrium, if H increases the OH- will
decrease (more H2O must be produced to balance
equilibrium) - If H decreases the OH- will increase (more
H2O will react to balance the equilibrium) - 1 x 10-14 H x OH-
33
- Acidic Solution One in which the H is
greater than the OH- - The H concentration is greater than 1 x 10-7
- Basic (Alkaline) Solution One in H is less
than OH- - The OH- concentration is greater than 1 x 10-7
- If the H in a solution is 1.0 x 10-5 M, is the
solution acidic, basic or neutral? - a) What is the OH- of this solution?
- 2) If the hydroxide ion concentration of an
aqueous solution is 1 x 10-3 M, what is the H
in the solution? - a) Is this solution acidic, basic or neutral?
34
pH The negative logarithm of the hydrogen-ion
concentration
- pH -logH
- A neutral solution has a H 1.0 x 10-7. What
is the pH of the neutral solution? - a) What is the pH of an acidic solution?
- b) What is the pH of a basic solution?
- Find the pH of each of the following solutions
- H 1 x 10-4 M
- H 0.0015 M
- H 1.0 x 10-12 M
- The pH of an unknown solution is 6.35. What is
its hydrogen-ion concentration? - What is the pH of a solution if OH- 4.0 x
10-11 M?
35
Indicator
- Its acid and base forms have different colors
36
NEUTRALIZATION REACTIONS
- The reaction between an acid and a base
- An acid and a base react in an aqueous solution
to produce a salt and water (which are both
neutral) - Salt Compound consisting of an anion from an
acid and a cation from a base
37
NEUTRALIZATION REACTIONS
- Write complete balanced equations for the
- following acid-base reactions.
- a) H2SO4(aq) KOH(aq) ?
- b) H3PO4(aq) Ca(OH)2(aq) ?
- c) HNO3(aq) Mg(OH)2 ?
38
TITRATION
- Adding a known amount of solution of known
concentration to determine the concentration of
another solution - Standard Solution The solution of known
concentration - End Point The point at which the indicator
changes color - Happens At The point of neutralization
39
TITRATION
- How to Calculate the Concentration from a
Titration - MaVa MbVb M Molarity
- V Volume
- Example A 25 mL solution of H2SO4 is completely
neutralized by 18 - mL of 1.0 M NaOH. What is the concentration of
the H2SO4 solution?