Section A: The Principles of Energy Harvest - PowerPoint PPT Presentation
Title: Section A: The Principles of Energy Harvest
1
(No Transcript)
2
Section A The Principles of Energy Harvest
- Cellular respiration and fermentation ?????? are
catabolic, energy-yielding ?????? ?????? ????
pathways - Cells recycle the ATP they use for work
- Redox reactions ??????? ???????-???????? release
energy when electrons move closer to
electronegative atoms - Electrons fall ????? from organic molecules to
oxygen during cellular respiration - 5. The fall of electrons during respiration is
stepwise ????????, via NAD and an Electron
Transport Chain
3
Respiration
4
??????? ????????????
??????? ??????
1- ??????? ???????
??????
3- ??????? ??????
????? ????????
2- ??????? ????????
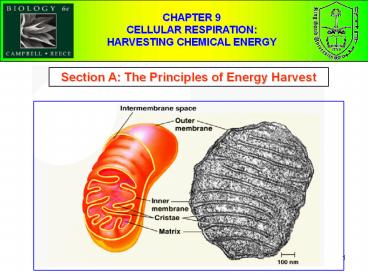
5
Fig. 9.1, Page 156
A)- Light energy
B)- Photosynthesis
1)- CO2 H2O
C)- Cellular respiration
ATP
Energy (heat)
6
1. Cellular respiration and fermentation are
catabolic, energy-yielding ????? ?????? pathways
- Organic molecules store energy in their
arrangement of atoms. - Enzymes catalyze the systematic degradation of
organic molecules that are rich in energy to
simpler waste products with less energy. - Some of the released energy is used to do work
and the rest is dissipated as heat. - Metabolic pathways that release the energy stored
in complex organic molecules are catabolic ????. - Fermentation is a type of catabolic process leads
to the partial degradation ?????? ?????? of
sugars in the absence of oxygen. - Cellular respiration is a more important
catabolic process, uses oxygen as a reactant to
complete the breakdown of a variety of organic
molecules. - This process is
- Organic compounds O2 -gt CO2 H2O Energy
- Carbohydrates, fats, and proteins can all be used
as the fuel, but we will start learning with
glucose. - C6H12O6 6O2 -gt 6CO2 6H2O Energy (ATP heat)
7
Cellular Respiration
Energy
Food (Fuel of energy)
Respiration
Cellular Activities
8
2. Cells recycle the ATP they use for work
- ATP (Adenosine Tri-Phosphate) is the important
molecule in cellular energetics ?????? ?????
??????. - The attachment of three negatively-charged
phosphate groups (P) is an unstable ??? ?????,
energy-storing ???? ?????? arrangement. - Loss of the end phosphate group release energ
- The price of most cellular work is the conversion
of ATP to ADP and phosphate (P). - An animal cell regenerates ???? ????? ATP from
ADP by adding P via the catabolism ??? of organic
molecules.
9
Adenosine Tri-Phosphate (ATP)
Adenosine
H2O
Triphosphate
Energy
P
Adenosine Di-Phosphate
Fig. 6.8, Page 94
10
The transfer of the terminal phosphate group from
ATP to another molecule is phosphorylation
???????????. This changes the shape
of the receiving molecule
in order to work
(transport, mechanical,
or chemical). When the phosphate groups leaves
the molecule, the molecule returns to its
original shape (stop).
11
How dose ATP drive cellular work ?
P
Microtubule
Organelle
P
Energy
Fig. 9.2, Page 157
12
3. Redox reactions release energy when electrons
move closer to electronegative atoms
- Catabolic pathways relocate ???? ????? the
electrons stored in food molecules, releasing
energy that is used to synthesize ?????? ATP. - Oxidation-reduction reactions (Redox reactions)
- Are reactions that result in the transfer of
one or more electrons from one reactant to
another - Oxidation
- Is the loss ???? of electrons.
- Reduction
- Is the addition ?????? of electrons.
Redox reactions require both a donor and
acceptor of e.
e-
Energy
13
4. Electrons fall from organic molecules to
oxygen during cellular respiration
- In cellular respiration, glucose and other fuel
molecules are oxidized, releasing energy. - Glucose is oxidized, oxygen is reduced, and
electrons loose potential energy. - H is the source of electrons that transfere to O.
- Thus, molecules that have an abundance of ???? ??
hydrogen are excellent fuels because their bonds
are a source of electrons that fall closer to
oxygen. - Enzymes lower the barrier of activation energy,
allowing these fuels to be oxidized slowly. - When H moves to O, it leaves bonds which
degenerated to release energy. - The resulting energy is used by the cell to
synthesis ATP .
Energy 686 kcal/mol
Energy
14
5. The fall of electrons ???????? ???????????
during respiration is stepwise ?????, by NAD
and an electron transport chain
- Cellular respiration does not oxidize glucose in
a single step that transfers all the hydrogen in
glucose to oxygen at one time. - Rather, glucose and other fuels are broken down
gradually ??????? in a series of steps, each
catalyzed by a specific enzyme. - At key steps ?? ??????? ????????, hydrogen atoms
move from glucose and passed first to the
coenzyme NAD (Nicotinamide Adenine
Dinucleotide). - Dehydrogenase enzymes strip two hydrogen atoms
from the fuel (e.g., glucose), pass two electrons
to NAD and release H.
15
- This changes the oxidized form, NAD, to the
reduced form NADH. - NAD functions as the oxidizing agent in many
of the redox steps during the catabolism of
glucose.
As electrons fall from NADH to oxygen, Their
energy is tapped to synthesize ATP.
Fig. 9.4
16
- Cellular respiration uses an electron transport
chain ????? ??? ???????????? to break ????????
the fall of electrons to O2 into several steps
??? ?????.
- The electron transport chain, consisting of
several molecules (primarily proteins), is built
into the inner membrane of a mitochondrion. - NADH takes electrons from food to the top of
the chain. - At the bottom, oxygen captures the electrons
and H to form water. - The free energy change from top to bottom is
-53 kcal/mole of NADH. - Electrons are passed by increasingly
electronegative molecules in the chain until they
are caught by oxygen (the most electronegative).
Fig. 9.5, Page 159
17
Summary of electron Fall steps during
respiration
- Falling of all H atoms from glucose to O is
gradually not at once.
- It occurs in steps, each one is catalyzed by an
enzyme.
- H atoms of glucose pass first to the co-enzyme
NAD to form NADH
- Then from NADH to electron transport chain, and
finally to O and releases energy to form ATP.
Energy
Page 158 Fig. 9.5