Flashback!! - PowerPoint PPT Presentation
1 / 21
Title:
Flashback!!
Description:
Which substance requires more heat to increase the temperature by 5 C? Specific heat capacity (C. p): amount of heat(q) required to raise 1 g of substance by 1 C – PowerPoint PPT presentation
Number of Views:72
Avg rating:3.0/5.0
Title: Flashback!!
1
Flashback!!
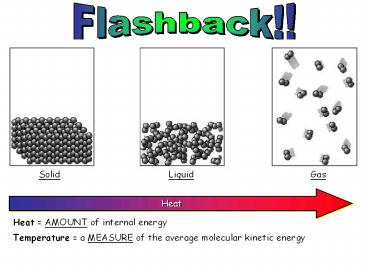
Heat
Heat AMOUNT of internal energy Temperature a
MEASURE of the average molecular kinetic energy
2
Both blocks are at the same temperature. Do they
both contain the same amount of heat?
3
Which substance requires more heat to increase
the temperature by 5 C?
Cp(Pb) 0.126 J/gC
Cp(paraffin) 2.1 J/gC
4
How much heat is required by the 100 g candle to
increase the temperature by 5 C?
Cp(paraffin) 2.1 J/gC
q Cp(mass)(DT)
q (2.1 J/gC)(100 g)(5 C)
q 1050 J
q Cp(mass)(DT)
1050 J (4.184 J/gC)(100g)(DT)
For the same amount of heat and mass, DT
decreases as the specific heat of the substance
increases
DT 2.5 C
5
If the temperature of the lead is 327C before it
hits the water, what is the final temperature of
the lead after hitting the water?
100 kg Pb
-qPb qH2O
200 ft
Tf 93 C
Ti 20 C
Cp(Pb) 0.13 J/gC
Baltimore Shot Tower http//www.baltimore.to/ShotT
ower/
Cp (H2O) 4.18 J/gC
6
Melting one 14-gram Al soda can requires 5.55 kJ
of energy. What is its molar heat of fusion?
105,000 cans are recycled in the US every
minute. How many kJ/s are being used in recycling
Al cans?
Thats equivalent to burning 2300 food Calories/s!
7
Experiment Heat two beakers containing 18 g of
water at the same rate, and monitor their
temperatures. Question Will their temperatures
increase at the same rate?
90 C
100 C
- 10 C
0 C
18 g H2O 1 mole H2O
8
(No Transcript)
9
Heating curve of water
Gas warming
100
Temperature (C)
liquid gas present
liquid warming
solid liquid present
0
solid warming
Heat (kJ/s)
10
Heating curve of water
100
Temperature (C)
boiling/condensation point
melting/freezing point
0
Heat (kJ/s)
Temperature is constant during phase
transitions!! All heat energy goes to changing
the state of matter.
11
Heating curve of water
100
Temperature (C)
DHvap
(heat of vaporization)
0
DHfus
(heat of fusion)
Heat (kJ/s)
DHfus the amount of heat needed to covert a
solid into its liquid phase
DHvap the amount of heat needed to convert a
liquid into its gaseous phase
12
Heating curve of water
100
Temperature (C)
And vice versa
0
A greater DHfus more time to melt
Heat (kJ/s)
13
- Heating Curve Wrap Up
- The specific heat capacity (Cp)of a substance
determines the temperature change observed when
heat is added or withdrawn from the substance. - Temperature is INVARIANT during phase
transitions. - The amount of heat required to convert one mole
of the substance from one phase to another is its
molar enthalpy of transition (DHfus, DHvap,
DHsub). - The amount of heat given off for one mole of a
substance during a phase transition while cooling
is its molar enthalpy of transition (DHcond,
DHsol, DHdep). - The shape of a heating curve depends upon the
heating rate, specific heat capacities of the
phases involved, and the enthalpies of transition.
What is the sign for all three?
DH
What is the sign for all three?
-DH
14
But how do we determine the heat content in the
first place?
DHrxn
Heat content of products heat content reactants
DHrxn lt 0
Reaction is exothermic
15
Heat of formation, DHf
- The DHf of all elements in their standard state
equals zero. - The DHf of all compounds is the molar heat of
reaction for synthesis of the compound from its
elements
DHf (AlBr3)
DHrxn 2DHf(AlBr3)
- Since the DHrxn can be used to find DHf, this
means that DHf can be used to find DHrxn WITHOUT
having to do all of the calorimetric measurements
ourselves!!
The Law of Conservation of Energy strikes again!!
16
DHrxn DHf(C6H12O6) 6 DHf(O2) 6 DHf(CO2)
6 DHf(H2O)
DHrxn -1250 kJ/mol 6(-393.5 kJ/mol)
6(-285.8 kJ/mol)
DHrxn 2825.8 kJ/mol
17
- Water will spontaneously evaporate at room
temperature even though this process is
endothermic. - What is providing the uphill driving force?
18
a measure of the disorder or randomness of the
particles that make up a system
- Water will spontaneously evaporate at room
temperature because it allows the disorder of the
water molecules to increase. - The entropy, S, of gases is gtgt than liquids or
solids. - If Sproducts gt Sreactants, DS is gt 0
Predict the sign of DS
DS lt 0
DS gt 0
19
Are all DS reactions spontaneous?
DS is large and positive
but DH is large and positive as well.
- Gibbs Free Energy, DG, allows us to predict the
spontaneity of a reaction using DH AND DS.
20
What is DG for this reaction at 25?C?
DHrxn SHf(products) SHf(reactants)
DSrxn SSf(products) SSf(reactants)
DSrxn 2(130.58 J/molK) 205.0 J/molK -
2(69.91 J/molK)
DGrxn DHrxn TDSrxn 571.66 kJ/mol -
298K(0.32634 kJ/molK)
DGrxn 474.41 kJ/mol
21
What is the minimum temperature needed to make
this reaction spontaneous?
DGrxn DHrxn TDSrxn 571.66 kJ/mol -
T(0.32634 kJ/molK)
Set DGrxn 0 to find minimum temperature
0 571.66 kJ/mol - T(0.32634 kJ/molK)
T (571.66 kJ/mol)/(0.32634 kJ/molK) 1751 K
T gt 1479 ?C