HIGHER CHEMISTRY REVISION. - PowerPoint PPT Presentation
1 / 4
Title:
HIGHER CHEMISTRY REVISION.
Description:
HIGHER CHEMISTRY REVISION. Unit 3 :- Nuclear Chemistry Polonium-210 is a radioisotope that decays by alpha-emission. The half life of polonium-210 is 140 days. – PowerPoint PPT presentation
Number of Views:51
Avg rating:3.0/5.0
Title: HIGHER CHEMISTRY REVISION.
1
HIGHER CHEMISTRY REVISION.
Unit 3 - Nuclear Chemistry
- Polonium-210 is a radioisotope that decays by
alpha-emission. - The half life of polonium-210 is 140
days.
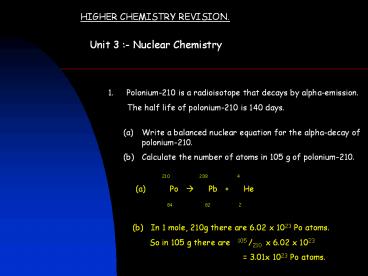
- Write a balanced nuclear equation for the
alpha-decay of polonium-210. - Calculate the number of atoms in 105 g of
polonium-210.
2
2. Carbon-14 dating can be used to estimate the
age of charcoal found in archaeological
sites. Carbon-14 has a half life of 5740
years. (a) Carbon-14 decays by beta emission.
Write a balanced equation for this decay. (b)
Why can carbon-14 not be used to estimate the age
of fossil fuels?
3. The grid shows the possible effect of
temperature change on reaction rate.
Identify the graph which shows how the
rate of reaction varies with temperature
in the radioactive decay of phosphorus-32.
A
B
C
E
F
D
3
4. Technetium-99, which has a long half-life, is
produced as a radioactive waste product in
nuclear reactors. One way of reducing the
danger of this isotope is to change it into
technetium-100 by bombardment with particles, as
shown by the nuclear equation. Tc X ?
Tc (a) Identify particle X. (b)
Technetium-100 decays by beta-emission.
Write a balanced nuclear equation for this
reaction. (c) Technetium-100 has a
half-life of 16 s. If a sample of
technetium-100 is left for 48 s, what fraction of
the sample would remain?
99 43
100 43
4
(No Transcript)