Atomic Theory and Structure PowerPoint PPT Presentation
Title: Atomic Theory and Structure
1
Atomic Theory and Structure
- what everything is made of
2
What is an atom?
- The smallest particle into which an element can
be divided and still be the same substance - The smallest unit of of a substance
- And when I say small, I mean small
- A penny has 20,000,000,000,000,000,000,000 atoms
(20 thousand billion billion)
3
If its so small, how was it discovered?
- Not long after Greece finally defeated Persia, a
Greek philosopher named Democritus did some
thinking - If you cut something in half, and then cut that
in half, and keep going, youll end up with
something you cant cut
4
Over 2000 years later
- John Dalton, a teacher, wondered why hydrogen and
oxygen always combine in the same way to make
water (a compound) - It must have something to do with how single
atoms interact
5
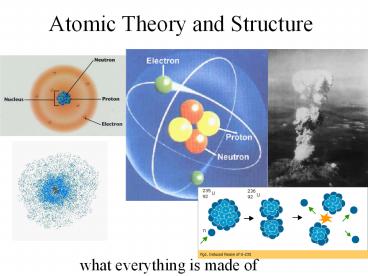
So, whats in an atom?
- Atoms have a nucleus
- Made of protons and neutrons
- The nucleus is very small, dense, and
positively-charged - Contains most of the atoms mass
- Outside the nucleus, electrons zip around in
electron clouds
6
Protons
- Particles in the atomic nucleus with a positive
() charge - We use the number of protons in an atoms nucleus
to determine what element it is - Atomic number
7
Neutrons
- A particle in the nucleus with NO charge
- Isotopes are atoms that have the same number of
protons, but different numbers of neutrons - Mass Number is the sum of protons and neutrons in
an atom - Atomic Mass is the average mass of an elements
isotopes
8
Electrons
- A particle outside the nucleus with a negative
(-) charge - Found within electron clouds
- Very small
- 1,800 electrons are about the size of one proton
- Their (-) charge cancels out the protons ()
charge - An atom with unequal protons and electrons is
called an ion - Either () or (-)
9
So what?
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.