The Nuclear Atom PowerPoint PPT Presentation
1 / 45
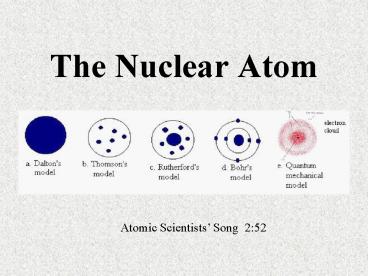
Title: The Nuclear Atom
1
The Nuclear Atom
Atomic Scientists Song 252
2
Aristotle (460 B.C. 370 B.C.)
- emphasized that nature consisted of four
elements air, earth, fire, and water - did not believe in discontinuous or separate
atoms, but felt that matter was continuous
3
Democritus (460 B.C. 370 B.C.)
- first to suggest the existence of atoms (Greek
word atomos indivisible) - atoms are indivisible and indestructible
- no experimental support
http//www.stenudd.com/myth/Greek/images/democritu
s_1628_Brugghen.jpg
4
John Dalton (1766-1844)
- used scientific method to test Democrituss ideas
- Daltons atomic theory
- elements composed of atoms
- atoms of the same element are alike
- different atoms can combine in ratios to form
compounds - chemical reactions can occur when atoms are
separated, joined, or rearranged (but atoms are
not created nor destroyed)
5
J.J. Thompson (1856-1940)(need to know this guy)
- discovered the electron
- thought atom was negative charges stuck in a
positive charged lump - referred to as the plumb pudding model
6
Robert A. Millikan (1868-1953)
- found the quantity of charge carried by an
electron (one unit of negative charge) - calculated the mass of an electron (1/1840th the
mass of a hydrogen atom)
7
Ernest Rutherford (1871-1937)(need to know this
guy)
Like howitzer shells bouncing off of tissue
paper!
- proposed that the atom is mostly empty space
- positive charges and almost of the mass are in a
small, centralized region called the nucleus
8
Rutherford Flash Animation
9
(No Transcript)
10
Try it Yourself!
In the following pictures, there is a target
hidden by a cloud. To figure out the shape of the
target, we shot some beams into the cloud and
recorded where the beams came out. Can you figure
out the shape of the target?
?
11
The Answers
Target 1
Target 2
12
Niels Bohr (1855-1962)(need to know this guy)
- electrons found only in specific circular paths
(orbits) around the nucleus - based on information about how the energy of an
atom changes when it absorbs and emits light - called these fixed energies energy levels
13
(No Transcript)
14
Erwin Schrodinger (1926)
- quantum mechanical model
- probability of electron locations around the
nucleus - not an exact orbit
- eventually became the electron cloud model
15
Schrödinger's Cat video 141
16
Werner Heisenberg (1927)(need to know this guy)
- Heisenberg Uncertainty Principle impossible to
know the exact position and momentum of an
electron at the same time - the observer affects the observed
http//www.deutsches-museum-bonn.de/ausstellungen/
heisenberg/bilder/heisenberg_2.jpg
17
(No Transcript)
18
(No Transcript)
19
(No Transcript)
20
(No Transcript)
21
(No Transcript)
22
Structure of the Atom
23
(No Transcript)
24
Particle Charge Mass (atomic mass units) Location
Proton 1 1 nucleus
Neutron Ø 1 nucleus
Electron - 1 5.0 x 10-4 (considered negligible) orbit, level, cloud
25
Carbon- 12 as a standard
- carbon- 12
- ALL masses on the periodic table are based on
their relationship to carbon-12 - the carbon- 12 isotope has been given the atomic
weight of exactly 12.000000000 and is used as the
basis upon which the atomic weight of other
isotopes is determined
26
Even smaller particles
- quarks
- make protons neutrons
- 6 types
27
(No Transcript)
28
(No Transcript)
29
(No Transcript)
30
Learning Check
- An atom has 14 protons and 20 neutrons.
- A. Its atomic number is
- 1) 14 2) 16 3) 34
- B. Its mass number is
- 1) 14 2) 16 3) 34
- C. The element is
- 1) Si 2) Ca 3) Se
- D. The number of electrons in a neutral atom
is - 1) 14 2) 6 3) 20
31
Isotopes
- same element but differ in their number of
neutrons - the atomic mass on periodic table is the WEIGHTED
AVERAGE MASS of all the isotopes of that
element - this is based on an isotopes natural abundance
- the percentage of each isotope of an element that
occurs in nature - have the same chemical properties (reactivity)
but different physical properties (density,
melting/boiling point)
32
(No Transcript)
33
(No Transcript)
34
(No Transcript)
35
2.3
36
(No Transcript)
37
Chemical symbols for isotopes
- two different ways to write isotopes
- example for sodium
- sodium- 23
- only shows mass number (23) of the sodium isotope
- 23 Na
- shows the mass number (23) and the atomic (11)
of the sodium isotope
11
A
Atomic Number
Z
38
Isotopes?
- Which of the following represent isotopes of the
same element? Which element? - 234 X 234 X 235 X 238 X
- 92 93 92 92
- 92 is the element
uranium
39
Do You Understand Isotopes?
6 protons, 8 (14 - 6) neutrons, 6 electrons
6 protons, 5 (11 - 6) neutrons, 6 electrons
40
Radioactive Isotopes (dont need to know)
- unstable isotopes that break down over time
- uses
- cobalt 60
- radiation treatment for cancer
- carbon 14
- used to date objects up to 60,000
- years old
- iodine 125 and iodine 131
- ingested and used for
- medical imaging
Dangerous, but worth the risk
41
The Mass Spectrometer
42
(No Transcript)
43
- has many applications, but one of the simplest is
to determine the natural abundances of the
isotopes of a particular element - the relative atomic mass can be calculated from
the data from the mass spectrometer
Mass spectrometer video (226) http//www.youtube.
com/watch?v_L4U6ImYSj0
44
(No Transcript)
45
Calculate the relative atomic mass of magnesium
with the provided data.
- magnesium results from the mass spectrometer
- 80 24Mg
- 10 25Mg
- 10 26Mg
- just a simple weighted mean
- .80(24) .10(25) .10(26) 24.3 amu