References PowerPoint PPT Presentation
Title: References
1
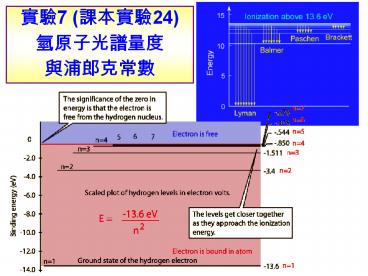
??7 (????24) ?????????????
2
References
- http//csep10.phys.utk.edu/astr162/lect/light/bohr
.html - ???????????????24??
3
?? ??????(atomic spectrum)
?? ???(H)?? (????) ?? En -(mee4/8e02h2)/n2 ??
n 1, E1 -13.6 eV ?????n ?, E? 0 DE
En1 En2 hf hc/l h 6.63 x 10-34 J-s c
3.00 x 108 m/s Balmer series lines (??????)
n?2 Visible spectrum (????) Ha 3?2, l 656.3 nm
Hb 4?2, l 486.1 nm Hg 5?2, l 434.0 nm
4
????
- ?????(H2)????,
- ???(H2)???????(n ? 1)??????(n?2)???????
- (spontaneous emission)
- (????,????)
- 2. ?????(spectrometer)
- ???????
- ???????,????????
- dsinq ml (m 0, ?1, ?2, )
- m 1 ???? (la, lb, lg)
- m 2 ??(lb)
- ????(q),??d?m,???(l)
- ??? l/Dl mN (N ???)
5
3. ?????? 1/l (mee4/8e0h3c)(1/22 - 1/n2)
(n?2) (1/RH)(1/4 - 1/n2) RH
8.31 J/mol-K (Rydberg constant) 4. ?????????RH,
?? me 9.11 x 10-31 kg e 1.60 x 10-19
C e0 8.85 x 10-12 F/m c 3.00 x 108
m/s ??????h, ???? h 6.63 x 10-34 J-s ??
5. ???????????????????
6
DVD ?? (The Atom)(The Mechanical Universeand
Beyond/MU49)
Annenberg/CPB/www.learner.org
Bohrs theory of hydrogen atom (???????) Potential
energy of electron bound to a proton U
-e2/4pe0r Total energy for circular orbit with
centrifugal force mv2/r e2/4pe0r2 E K U
mv2/2 U - e2/8pe0r Frequency condition from
spectral line (??????) DE Ei Ef hfif
Quantized angular momentum L of the orbiting
electron (???????) L mvr n(h/2p) n
1, 2, 3, Radii of allowed orbits r
n2h2e0/pme2 n2rB (rB 0.0529 nm 52.9 pm
Bohrs radius) Allowed energy states En
-(mee4/8e02h2)/n2 -13.6 eV/n2
7
Importance of the Hydrogen Atom
- The H-atom is the only atomic system that can be
solved exactly. - Much of what was learned about the H-atom, with
its single electron, can be extended to such
single-electron ions as He and Li2. - The H-atom proved to be an ideal system for
performing precision tests of theory against
experiment. - Also for improving our understanding of atomic
structure. - The quantum numbers that are used to characterize
the allowed states of hydrogen can also be used
to investigate more complex atoms. This allows us
to understand the periodic table. - The basic ideas about atomic structure must be
well understood before we attempt to deal with
the complexities of molecular structures and the
electronic structure of solids.
8
J. J. Thomson Atomic Model Early Model
(Newtons Time) of the Atom
- The atom was a tiny, hard indestructible sphere.
- It was a particle model that ignored any internal
structure. - The model was a good basis for the kinetic theory
of gases.
- J. J. Thomson established the charge to mass
ratio for electrons. - His model of the atom
- A volume of positive charge.
- Electrons embedded throughout the volume.
9
Rutherfords Thin Foil Experiment
- Experiments done in 1911.
- A beam of positively charged alpha particles hit
and are scattered from a thin foil target. - Large deflections could not be explained by
Thomsons model.
- Rutherford
- Planetary model based on results of thin foil
experiments - Positive charge is concentrated in the center of
the atom, called the nucleus. - Electrons orbit the nucleus like planets orbit
the sun
10
Difficulties with the Rutherford Model
- Atoms emit certain discrete characteristic
frequencies of electromagnetic radiation. - The Rutherford model is unable to explain this
phenomena. - Rutherfords electrons are undergoing a
centripetal acceleration.
- It should radiate electromagnetic waves of the
same frequency. - The radius should steadily decrease as this
radiation is given off. - The electron should eventually spiral into the
nucleus. - ? But the fact doesnt.
11
The Bohr Theory of Hydrogen-A Planetary Model of
the Atom
- In 1913 Bohr provided an explanation of atomic
spectra that includes some features of the
currently accepted theory.
- His model includes both classical and
non-classical ideas. - He applied Plancks ideas of quantized energy
levels to orbiting electrons. - In this model, the electrons are generally
confined to stable, nonradiating orbits called
stationary states. - Used Einsteins concept of the photon to arrive
at an expression for the frequency of radiation
emitted when the atom makes a transition.
12
- The Bohr Model is probably familiar as the
"planetary model" of the atom. - for example, is used as a symbol for atomic
energy (a bit of a misnomer, since the energy in
"atomic energy" is actually the energy of the
nucleus, rather than the entire atom). - In the Bohr Model the neutrons and protons
(symbolized by red and blue balls in the adjacent
image) occupy a dense central region called the
nucleus, and the electrons orbit the nucleus much
like planets orbiting the Sun (but the orbits are
not confined to a plane as is approximately true
in the Solar System). - The adjacent image is not to scale since in the
realistic case the radius of the nucleus is about
100,000 times smaller than the radius of the
entire atom, and as far as we can tell electrons
are point particles without a physical extent.
13
- This similarity between a planetary model and the
Bohr Model of the atom ultimately arises - because the attractive gravitational force in
a solar system and - ? the attractive Coulomb (electrical) force
between the positively charged nucleus and the
negatively charged electrons in an atom are
mathematically of the same form. - The form is the same, but the intrinsic strength
of the Coulomb interaction is much larger than
that of the gravitational interaction - in addition, there are positive and negative
electrical charges so the Coulomb interaction can
be either attractive or repulsive, but
gravitation is always attractive in our present
Universe.
14
The Orbits Are Quantized-Quantized energy levels
in hydrogen
- The basic feature of quantum mechanics that is
incorporated in the Bohr Model. - That is completely different from the analogous
planetary model is that the energy of the
particles in the Bohr atom is restricted to
certain discrete values. - One says that the energy is quantized.
- This means that only certain orbit
- with certain radii are allowed
- orbits in between simply
- don't exist.
15
(No Transcript)
16
Quantized energy levels in hydrogen
17
Quantized Energy Levels in the hydrogen atom
- These energy levels are labeled by an integer n
that is called a quantum number. - The lowest energy state is generally termed the
ground state. - The states with successively more energy than the
ground state are called the first excited state,
the second excited state, and so on. - Beyond an energy called the ionization potential
the single electron of the hydrogen atom is no
longer bound to the atom. - Then the energy levels form a continuum.
- In the case of hydrogen, this continuum starts at
13.6 eV above the ground state ("eV" stands for
"electron-Volt", a common unit of energy in
atomic physics).
18
Atomic Excitation and De-excitation
- Atoms can make transitions between the orbits
allowed by quantum mechanics by absorbing or
emitting exactly the energy difference between
the orbits.
Excitation by absorption of light and
de-excitation by emission of light
19
- Atoms can make transitions between the orbits
allowed by quantum mechanics by absorbing or
emitting exactly the energy difference between
the orbits. - In each case the wavelength of the emitted or
absorbed light is exactly such that the photon
carries the energy difference between the two
orbits. - This energy may be calculated by dividing the
product of the Planck constant and the speed of
light hc by the wavelength of the light). - Thus, an atom can absorb or emit only certain
discrete wavelengths (or equivalently,
frequencies or energies). - Here is a Shockwave movie of atomic absorption
and emission in - Here is a Java applet illustrating atomic
absorption and emission.
20
Separation of light by a prism according to
wavelength
- Based on the Bohr atom, isolated atoms can absorb
and emit packets of electromagnetic radiation
having discrete energies dictated by the detailed
atomic structure of the atoms. - When the corresponding light is passed through a
prism or spectrograph it is separated spatially
according to wavelength ?.
21
Continuum, Emission Absorption Spectra
- The corresponding spectrum may exhibit a
continuum, or may have superposed on the
continuum bright lines (an emission spectrum) or
dark lines (an absorption spectrum), as
illustrated in the following figure.
22
Origin of Continuum, Emission Absorption Spectra
- The emission spectra are produced by thin gases
in which the atoms do not experience many
collisions (because of the low density). - The emission lines correspond to photons of
discrete energies that are emitted when excited
atomic states in the gas make transitions back to
lower-lying levels. - A continuum spectrum results when the gas
pressures are higher. Generally, solids, liquids,
or dense gases emit light at all wavelengths when
heated. - An absorption spectrum occurs when light passes
through a cold, dilute gas and atoms in the gas
absorb at characteristic frequencies since the
re-emitted light is unlikely to be emitted in the
same direction as the absorbed photon, this gives
rise to dark lines (absence of light) in the
spectrum.
23
Sources of continuous, emission, and absorption
spectra
- The emission spectra are produced by thin gases
in which the atoms do not experience many
collisions (because of the low density). - The emission lines correspond to photons of
discrete energies that are emitted when excited
atomic states in the gas make transitions back to
lower-lying levels. - An absorption spectrum occurs when light passes
through a cold, dilute gas and atoms in the gas
absorb at characteristic frequencies since the
re-emitted light is unlikely to be emitted in the
same direction as the absorbed photon, this gives
rise to dark lines (absence of light) in the
spectrum.
24
Hydrogen Emission Absorption Series
(visible light)
Hydrogen emission series
(UV spectrum)
25
Hydrogen Emission Absorption Series
- The spectrum of hydrogen is particularly
important in astronomy because most of the
Universe is made of hydrogen. - Emission or absorption processes in hydrogen give
rise to series, which are sequences of lines
corresponding to atomic transitions, each ending
or beginning with the same atomic state in
hydrogen. - The Balmer Series involves transitions starting
(for absorption) or ending (for emission) with
the first excited state of hydrogen. - The Lyman Series involves transitions that start
or end with the ground state of hydrogen. - Because of the details of hydrogen's atomic
structure, - the Balmer Series is in the visible spectrum and
- the Lyman Series is in the the UV.
26
- Because of the details of hydrogen's atomic
structure, - the Balmer Series is in the visible spectrum and
- the Lyman Series is in the the UV.
- The Balmer lines are designated by H with a greek
subscript Hi in order of decreasing wavelength. - Thus the longest wavelength Balmer transition is
designated H with a subscript alpha, H?. - the second longest H with a subscript beta, H?,
- and so on, H?, H?.
27
Electron Transitions
- An electron transition in hydrogen between
quantized energy levels with different quantum
numbers n yields a photon by emission with
quantum energy
This is often expressed in terms of the inverse
wavelength or "wave number" as follows
28
Quantized Energy States
- The electrons in free atoms can will be found in
only certain discrete energy states. These sharp
energy states are associated with the orbits or
shells of electrons in an atom, e.g., a hydrogen
atom. One of the implications of these quantized
energy states is that only certain photon
energies are allowed when electrons jump down
from higher levels to lower levels, producing the
hydrogen spectrum. The Bohr model successfully
predicted the energies for the hydrogen atom, but
had significant failures that were corrected by
solving the Schrodinger equation for the hydrogen
atom.
29
Hydrogen Energy Levels
30
Basic Structure of the Hydrogen Energy Levels
- It can be calculated from the Schrodinger
equation. - The energy levels agree with the earlier Bohr
model, and agree with experiment within a small
fraction of an electron volt. - If you look at the hydrogen energy levels at
extremely high resolution, you do find evidence
of some other small effects on the energy. - The 2p level is split into a pair of lines by the
spin-orbit effect. - The 2s and 2p states are found to differ a small
amount in what is called the Lamb shift. - And even the 1s ground state is split by the
interaction of electron spin and nuclear spin in
what is called hyperfine structure.
31
Balmer Line Seriesin Visible Spectrum
Wavelength (nm) Color
656.2 red
486.1 blue
434.0 blue-violet
410.1 violet
- 1885 - Johann Jacob Balmer
- Analyzed the hydrogen spectrum and found that
hydrogen emitted four bands of light within the
visible spectrum - Balmer found that the data fit to the following
equation - ? wavelength (nm)
- RH Rydberg's constant
- 1.09678 x 10-2 nm-1
- n1 the lower energy level
- n2 the higher energy level
32
(No Transcript)
33
Each series is named after its discoverer
- The Lyman series is the wavelengths in the ultra
violet (UV) spectrum of the hydrogen atom,
resulting from electrons dropping from higher
energy levels into the n 1 orbit. - The Balmer series is the wavelengths in the
visible light spectrum of the hydrogen atom,
resulting from electrons falling from higher
energy levels into the n 2 orbit. - The Paschen series is the wavelengths in the
infrared spectrum of the hydrogen atom, resulting
from electrons falling from higher energy levels
into the n 3 orbit. - The Brackett series is the wavelengths in the
infrared spectrum of the hydrogen atom, resulting
from electrons falling from higher energy levels
into the n 4 orbit. - The Pfund series is the wavelengths in the
infrared spectrum of the hydrogen atom, resulting
from electrons falling from higher energy levels
into the n 5 orbit.
34
Absorption Spectrum
- 1814 - Joseph von Fraunhofer
- Studied the absorption spectrum of the light
given off by the sun. - Absorption Spectrum - The spectrum of dark lines
against a light background that results from the
absorption of selected frequencies of the
electromagnetic radiation by an atom or molecule.
The Balmer Series of Hydrogen consists of four
visible lines.
35
The Balmer Series of Hydrogen (H) consists of
four visible lines
- The helium (He) spectrum is somewhat more complex
than that of hydrogen.
36
The neon (Ne) spectrum is dominated by red lines.
- The sodium (Na) spectrum consists of one very
bright yellow line.
37
The mercury (Hg) spectrum
38
(No Transcript)