eSubmission Roadmap (reflecting final version 1.0 dated 140721) - PowerPoint PPT Presentation
eSubmission Roadmap (reflecting final version 1.0 dated 140721)
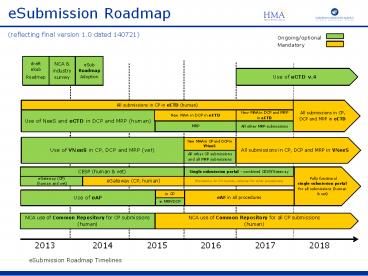
(reflecting final version 1.0 dated 140721) Ongoing/optional Mandatory draft eSub Roadmap NCA & industry survey eSub Roadmap Adoption Use of eCTD v.4 – PowerPoint PPT presentation
Title: eSubmission Roadmap (reflecting final version 1.0 dated 140721)
1
eSubmission Roadmap(reflecting final version 1.0
dated 140721)
Ongoing/optional Mandatory
draft eSub Roadmap
NCA industry survey
eSub Roadmap Adoption
Use of eCTD v.4
All submissions in CP, DCP and MRP in eCTD
All submissions in CP in eCTD (human)
Use of NeeS and eCTD in DCP and MRP (human)
New MAA in DCP in eCTD
New MAA in DCP and MRP in eCTD
MRP
All other MRP submissions
Use of VNeeS in CP, DCP and MRP (vet)
All submissions in CP, DCP and MRP in VNeeS
New MAA in CP and DCP in VNeeS
All other CP submissions and all MRP submissions
Fully functional single submission portal for
all submissions (human vet)
CESP (human vet)
Single submission portal - combined CESP/Gateway
eGateway (CP) (human and vet)
eGateway (CP, human)
(Mandatory for CP-human, optional for other
procedures)
Use of eAF
in CP
eAF in all procedures
in MRP/DCP
NCA use of Common Repository for all CP
submissions (human)
NCA use of Common Repository for CP submissions
(human)
2013 2014 2015 2016 2017 2018
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.