THE ELECTROLYSIS OF WATER PowerPoint PPT Presentation
Title: THE ELECTROLYSIS OF WATER
1
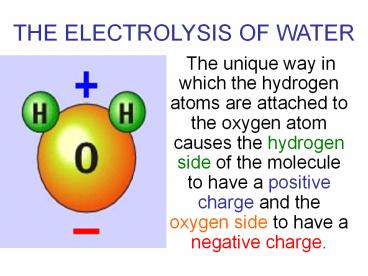
THE ELECTROLYSIS OF WATER
- The unique way in which the hydrogen atoms are
attached to the oxygen atom causes the hydrogen
side of the molecule to have a positive charge
and the oxygen side to have a negative charge.
2
- The Hydrogen atoms are attracted to the
- - charged terminal, and are pulled off the
- H2O molecule, filling the right tube in this
diagram with Hydrogen gas.
3
- The - Oxygen atoms are attracted to the
- charged terminal, and are pulled from the
- H2O molecule, filling the left tube in this
diagram with Oxygen gas.
4
- H2O consists of 2 H atoms and 1 O atom so twice
as much Hydrogen gas will be produced
5
2H2O ? 2H2 O2
- Faraday's 1st Law of Electrolysis - The mass of a
substance altered at an electrode during
electrolysis is directly proportional to the
quantity of electricity transferred at that
electrode. Quantity of electricity refers to the
quantity of electrical charge, typically measured
in coulomb. - IN OTHER WORDS Stronger battery more
electricity more gas produced (and faster, too)
6
Faradays laws continued
- Faraday's 2nd Law of Electrolysis - For a given
quantity of electricity (electric charge), the
mass of an elemental material altered at an
electrode is directly proportional to the
element's equivalent weight. - IN OTHER WORDS Since there are twice as many
Hydrogen atoms as Oxygen atoms in 2H2O, TWICE as
much HYDROGEN gas will be produced as Oxygen gas
7
- IS THIS A PHYSICAL OR A CHEMICAL CHANGE?
- WHAT EVIDENCE SUGGESTS THIS?
8
- 2H2O ? 2H2 O2
- WHAT DOES THE FORMULA ABOVE SUGGEST ABOUT THE
REACTION? - HOW CAN WE BE SURE?
9
- HOW DO WE IDENTIFY THE GAS????
10
FLAME AND GLOWING CINDER TESTS
11
- HYDROGEN GAS CAN EXPLODE WITH A POP, AND CAN
EMIT A BRIEF FLASH OF LIGHT. - SHOULD WE TRY IT?????
12
- Are you SURE?????
- Its REALLY DANGEROUS!!!!
- Its VERY LOUD AND SCARY!!!!
- ARE YOU SURE???
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.