Chap. 3 (Sec. 3-5 to End of Chapter) PowerPoint PPT Presentation
1 / 23
Title: Chap. 3 (Sec. 3-5 to End of Chapter)
1
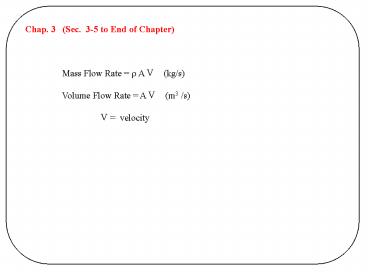
Chap. 3 (Sec. 3-5 to End of Chapter) Mass
Flow Rate r A V (kg/s) Volume Flow Rate
A V (m3 /s) V velocity
2
Conservation of Mass (Continuity Principle)
min
mout
3
Conservation of Mass (Continuity
Principle) Steady state ? Change of Any quantity
with time 0
(r A V)in (r A V)out Incompressible fluid ?
4
Flow Work Wflow PV wflow Pv Total energy of
moving fluid e Pv u p.e k.e
Pv h p.e k.e
5
- First Law of Thermodynamics (ENERGY
BALANCE) also known as Conservation of Energy
Principle - CLOSED SYSTEMS
- OPEN SYSTEMS (CONTROL VOLUME)
- STEADY FLOW
- UNSTEADY FLOW
6
CLOSED SYSTEMS (e.g piston-cylinder etc.)
Ein Eout ?Esystem
Net Energy transfer by heat, work, and mass
Change in internal, KE, PE etc
Q W ?E Qnet, in Wnet,out ?Esystem
Moving boundary work, Shaft work, Paddle Work etc
7
Q W ?E Qnet, in Wnet,out ?Esystem
Moving boundary work, Shaft work, Paddle Work etc
How the above equation simplifies for different
situations ? for stationary systems for
constant volume process for constant pressure
process for many other situations given in your
text book
8
Q W ?E Qnet, in Wnet,out ?Esystem
Moving boundary work, Shaft work, Paddle Work etc
For example Based on problem statement if you
having the following information Closed
System Stationary Adiabatic Constant Volume
Process Paddle Work (wnet, in) Final
Temperature ?
9
Wnet,in ?Usystem wnet,in ?usystem
What will be my approach if (a) Substances like
Steam, R134a if (b) ideal gases
10
if (a) Substances like Steam, R134a ?U m
(u2-u1)
As long as you have any two properties, you can
find rest How? ? State (Saturated liquid ,
mixture, superheated)
11
if (b) ideal gases ?U m (u2-u1) m Cv (T2
T1) PV mRT Pv RT (PV/T)1 (PV/T)2
12
STEADY FLOW SYSTEMS
Rate of Net Energy transfer out by heat, work,
and mass
Rate of Net Energy transfer in by heat, work,
and mass
Mass balance ?
13
One inlet (1) and one exit (2)
On a unit mass basis
14
In general, how above equation simplifies
for (how to judge - based on
function) Nozzles Diffusers Compressors,
Pumps Turbines Mixtures, Heat
Exchangers Throttling devices
15
UNSTEADY FLOW SYSTEMS Mass Balance
min mout ?msystem
?min ?mout (m2-m1)system
16
Energy Balance
Ein Eout ?Esystem
17
Chap. 5 The Second Law of Thermodynamics (What
is second law and how it helps)
Statements (in words or schematic) Some
concepts Heat Engine, Refrigerator, Heat Pump
Reversible and Irreversible processes The
Carnot Cycle and its importance
18
1ST LAW Qnet Wnet ?E for cyclic devices
?E 0 Therefore Wnet Qnet Wnet Qin -
Qout
19
HIGH TEMPERATURE RESERVOIR AT TH
?
QH
? Wnet, out
HE
QL
? LOW TEMPERATURE SINK AT TL
20
WARM ENVIRONMENT AT THgtTL (HOME)
?
WARM ENVIRONMENT AT THgtTL ?
QH
QH
?Wnet, IN
?Wnet, IN
HEAT PUMP
REFRI.
? COLD REFRIGERATED SPACE AT TEMPERATURE TL
? COLD ENVIRONMENT AT TEMPERATURE TL
QL
QL
21
CARNOT PRINCIPLES 1. The efficiency of an
irreversible heat engine is always less than of
a reversible one operating between the same two
reservoirs. 2. The efficiencies of all
reversible heat engines operating between the
same two reservoirs are the same (Independent
of working fluid and its properties, the way
cycle is executed, or the type of reversible
engine)
22
FOR REVERSIBLE PROCESS ALONE
23
(No Transcript)