Relating Electron Configuration to the Periodic Table PowerPoint PPT Presentation
Title: Relating Electron Configuration to the Periodic Table
1
Relating Electron Configuration to the Periodic
Table
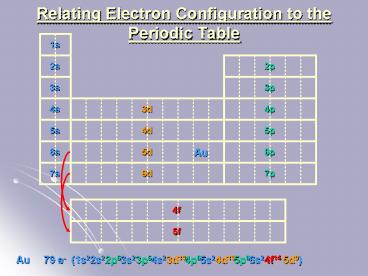
1s
2s
2p
3s
3p
4s
4p
3d
5s
5p
4d
6s
6p
5d
Au
7s
7p
6d
4f
5f
Au
(1s2
79 e-
2s2
2p6
3s2
3p6
4s2
3d10
4p6
5s2
4d10
5p6
6s2
4f14
5d9)
2
Predict the Electron Configurations of the
Following
- Example
P
P (1s22s22p63s23p3)
Co
Co (1s22s22p63s23p64s23d7)
Sr
Sr (1s22s22p63s23p64s23d104p65s2)
O
O ( He 2s22p4)
Fr
Fr ( Rn 7s1)
Am
Am ( Rn 7s25f7)
Core notation use noble gas to represent core
electrons
3
Electron Configurations of Negative Ions
- For negative ions add electrons to the last
unfilled shell.
P
P (1s22s22p63s23p3)
P3- (1s22s22p63s23p6)
P3-
O ( He 2s22p4)
O
O-
O- ( He 2s22p5)
O2-
O2- ( He 2s22p6)
4
Electron Configurations of Positive Ions
- For positive ions there are 2 rules
- e- in the outermost shell (largest n-value) are
removed first. - If there are e- in both the s- p- orbitals in
the outermost shell, remove the p-orbital e-
first.
K
K (1s22s22p63s23p64s1)
K
K (1s22s22p63s23p6 )
V (1s22s22p63s23p64s23d3)
V
V2
V2 (1s22s22p63s23p6 3d3)
Sn ( Kr 5s24d105p2)
Sn
Sn2
Sn2 ( Kr 4d10 )
5s2
Sn4
Sn4 ( Kr 4d10 )
5
What do the orbitals look like?
6
p - orbitals
d - orbitals
7
f - orbitals
8
Valence Electrons
- Valence electrons are the electrons in the
outermost s- p-orbitals
K
K (1s22s22p63s23p6 )
4s1
1 valence e-
V
V (1s22s22p63s23p6 3d3)
4s2
2 valence e-
Sn
Sn ( Kr 4d10 )
5s2
5p2
4 valence e-
9
Periodic Trends
- Atomic Radius
- Decreases from left to right across a period(due
to increased charge on the nucleus) - Increases from top to bottom in a group or
family(due to shielding effects larger
orbitals) - Electronegativity
- The attraction an atom has for a shared pair of
electrons in a covalent bond - Increases from left to right across a period
- Decreases from top to bottom in a group or
family
10
- Ionization Energy
- The energy needed to remove an electron.
- Increases from left to right across a period
- Decreases from top to bottom in a group or family
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.