Applications of Redox Titrations - PowerPoint PPT Presentation
1 / 12
Title:
Applications of Redox Titrations
Description:
Cerium(IV) solutions. Ce4 1e- Ce3 Eo' = 1.44 V in 1 M H2SO4 ... Some applications of KMnO4 and Cerium(IV) in acid solutions. Applications of Redox Titrations ... – PowerPoint PPT presentation
Number of Views:1842
Avg rating:5.0/5.0
Title: Applications of Redox Titrations
1
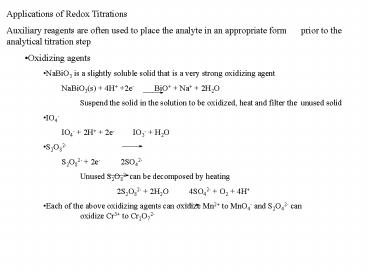
- Applications of Redox Titrations
- Auxiliary reagents are often used to place the
analyte in an appropriate form prior to the
analytical titration step - Oxidizing agents
- NaBiO3 is a slightly soluble solid that is a very
strong oxidizing agent - NaBiO3(s) 4H 2e- BiO Na 2H2O
- Suspend the solid in the solution to be oxidized,
heat and filter the unused solid - IO4-
- IO4- 2H 2e- IO3- H2O
- S2O82-
- S2O82- 2e- 2SO42-
- Unused S2O82- can be decomposed by heating
- 2S2O82- 2H2O 4SO42- O2 4H
- Each of the above oxidizing agents can oxidize
Mn2 to MnO4- and S2O42- can oxidize Cr3 to
Cr2O72-
2
- Applications of Redox Titrations
- Auxiliary reagents ...
- Reducing agents
- Walden reductor consists of a gravity-chromatograp
hic-column like tube filled with Ag and a
solution of HCl - Ag Cl- AgCl 1e-
- Jones reductor uses similar glassware filled with
amalgamated zinc - 2Zn Hg2 Zn(Hg)(s) Zn2 preparation
of amalgam - Zn(Hg) Zn2 Hg 2e- oxidation of
zinc amalgam - The Hg prevents the oxidation of Zn by H and
solutions containing moderate concentrations of
H can pass through the reductor without undue
contamination of the analytical solution by Zn2
3
Applications of Redox Titrations Uses of Walden
and Jones reductors
- Other reducing agents can be used including Sn,
Sn(II), Al, Cd, Ni, Pb, Cu, Ag(Cl-) - Most methods use oxidizing agents as titrants
- MnO4-, Ce(IV), Cr2O72-, I2, I3-
4
- Applications of Redox Titrations
- MnO4- is the most used oxidizing agent titrant
- MnO4- 8H 5e- Mn2 4H2O Eo 1.51
V in acid - Self-indicating but endpoint fades due to
- 2MnO4- 3Mn2 5MnO2 4H K 1046 but
slow - MnO4- 4H 3e- MnO2 2H2O if pH gt 4,
neutral or basic - MnO4- is unstable as it oxidizes water
- 4MnO4- 2H2O 4MnO2 3O2 4OH- K gtgt 1
but slow - Reaction catalyzed by light, heat, acids,
Mn(II), and MnO2 - Solutions containing excess MnO4- should not be
heated because of this decomposition reaction
which cannot be corrected for by using a blank - Warm solutions can be titrated with MnO4-,
however - Preparation of KMnO4 solutions
- Cannot prepare standard solutions by direct
weighing as KMnO4 is contaminated with MnO2 - Prepare solutions hot and allow to stand
overnight, then filter using Gooch crucible to
remove the MnO2 - Store in a dark place except when in use
5
- Applications of Redox Titrations
- MnO4- is the most used oxidizing agent titrant
- Standardization of MnO4- solutions
- Primary standard Na2C2O4 in acid is used
- H2C2O4 2CO2 2H 2e-
- reaction is rapid in hot solution but catalyzed
by Mn2, so the initial MnO4- takes several
seconds to react and discharge the color - Method of McBride - slowly titrate a 60 oC
solution - Low results are due to O2 H2C2O4 H2O2
CO2 - Method of Fowler and Bright - add 95 of
required MnO4- to a cold H2C2O4 solution, heat
to 60 oC and finish the titration - Other standards include As2O3, Fe, KI
6
- Applications of Redox Titrations
- MnO4- is the most used oxidizing agent titrant
- Analysis of iron alloys and iron ores with MnO4-
- Dissolve in HCl - Fe(II) forms a stable complex
with Cl- which enhances the dissolution process - Often the resulting solution consists as a
mixture of Fe(II) and Fe(III) - Use a Jones reductor to reduce Fe(III) to Fe(II),
but other metal impurities may be reduced that
would react with MnO4- - Use a Walden reductor which does not reduce
Cr(III) or Ti(IV) but V species could interfere - Use SnCl2 Sn2 2Fe3 Fe2 Sn4
- remove excess Sn2 Sn2 Hg2 2Cl-
Sn4 Hg2Cl2(s) - avoid too much HgCl2 Sn2 Hg2 Hg
Sn4 - Analysis of Ca2
- Precipitate as CaC2O4 and dissolve in acid
- Titrate the H2C2O4 with MnO4-
7
- Applications of Redox Titrations
- Cerium(IV) solutions
- Ce4 1e- Ce3 Eo 1.44 V in 1 M
H2SO4 - Primary standard grade Ce(NO3)42NH4NO3 can be
used to prepare standard solutions in solutions
at least 0.1 M in H2SO4 - Other reagents in reagent-grade quality can be
used and the resulting solutions standardized by
titration of primary standard grade sodium
oxalate in 1M H2SO4 at 50 oC - Ce(IV) solutions are indefinitely stable even at
100 oC - Solutions of Ce(IV) with the same oxidizing
capacity as MnO4- solutions cost 50 times as
much as the MnO4- solutions - Ce(IV) unlike MnO4- will not oxidize Cl- in HCl
solutions, so solutions prepared with HCl can be
used
8
Applications of Redox Titrations Some
applications of KMnO4 and Cerium(IV) in acid
solutions
9
- Applications of Redox Titrations
- Iodimetry and Iodometry
- Iodimetry is the direct use of I2 or I3-
solutions - Saturated I2(aq) is 0.001 M, so I2(s) is added to
a small volume of concentrated KI and diluted
to form I3- - I3- solutions are not very stable and must be
standardized every few days - 4I- O2 4H 2I2 2H2O solution
increases I2 concentration - I3- is a weak oxidizing agent so steps are taken
to enhance its potential - Adjust pH
- pH does not affect the potential of the I3-/I-
couple but - H3AsO4 2H 2e- H3AsO4 H2O
- Form complexes
- Fe(III) can be made to form EDTA complexes under
conditions which Fe(II) does not form complexes
thus enhancing the reaction of Fe(III) with I3- - 2Fe2 EDTA I3- 2FeY- 3I-
- In alkaline solutions I2 OH- HOI
I- - 3HOI 3OH- IO3-
2I- 3H2O
10
- Applications of Redox Titrations
- Iodimetry
- End points use I3- since the eye can detect 5 x
10-6 M I3- - Use an immiscible organic liquid to extract the
I2 - Use starch
- Standardization of I3- solutions
- Dissolve As2O3 in 1M NaOH and neutralize excess
base with HCl to prevent oxidation of As(III) to
As(IV) - H3AsO3 I3- H2O H3AsO4 3I- 2H K
0.16 - Keep pH 7 - 8 with CO2/HCO3- buffer to draw
reaction forward - Iodometry is the indirect use of I2 of I3-
produced by the reaction of I- added to a
solution containing an oxidizing analyte. - The I(0) is titrated with standardized S2O32-
- 2S2O32- I3- S4O62- 3I- note 1e-
per S2O32- 2 S2O32- S4O62- 2e- - Other oxidizers form SO42- 4HOI S2O32-
H2O 2SO42- 4I- 6H - HOI produced when I3- solution becomes basic
11
- Applications of Redox Titrations
- Iodometry
- S2O32- solutions
- pH must be controlled S2O32- H
HS2O3- - HS2O3- S(s) HSO3-
- If pH low, reaction takes seconds
- Stabilize with Na2CO3 or borax pH7-9
- Must neutralize OH- by acidfying I3- solution to
prevent formation of HOI - Certain bacteria should be excuded - sterilize
the solution by boiling the water - Sunlight can cause decomposition of S2O32-
solutions - Atospheric O2 can oxidize the S2O32-
- Standardization of S2O32- solutions
- Use a reagent that produces a known amount of I3-
and titrate the I3- - IO3- 0.5I- 6H 5e- 0.5I3-
3H2O - 7.5I-
2.5I3- 5e- - IO3- 8I- 6H 3I3-
3H2O Obtain 6 mol I(0)/mol IO3-
12
- Applications of Redox Titrations
- Iodometry
- Errors
- Air oxidation of I- 6I- O2 4H 2I3-
2H2O - Reaction is slow catalyzed by light, high H
concentration and Cu(II) - Volatalization of I2 maintain large excess of
I-, titrate rapidly, keep solutions cool - Decomposition of S2O32-
- Avoid basic solutions so HOI not produced and
S2O32- will not go to SO42- - Do not add starch too early as high
concentrations of I3- will decompose it