Solar%20Cell PowerPoint PPT Presentation
Title: Solar%20Cell
1
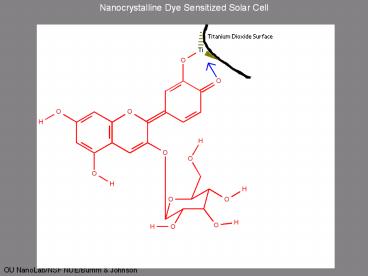
Nanocrystalline Dye Sensitized Solar Cell
2
Outline
- Motivation
- History
- Cell Schematic
- Useful Physics
- Construction Procedure
- Preparation and deposition of TiO2 (10-50 nm
diameter) - Preparation of dye and staining semiconducter
- Carbon Coating counter-electrode
- Assemblage
- Electric Output
- Data Analysis
- Conclusion
3
Motivation
- Economically feasible harnessing of solar energy
- Reduce fossil fuel usage and subsequent pollution
- Provide usable energy to inaccessible and
economically challenged communities - Modeling of biological photochemical systems
- Improvement of current photographic methods
4
History
- 1839 French physicist Antoine-Cesar Becquerel
observed that shining light on an electrode
submerged in electrolyte would create an electric
current. - 1941 American Russell Ohl invented a PN junction
silicon solar cell - The dye sensitized solar cell was developed in
1992 by Graetzel (EPFL, Laussane, Switzerland)
and utilizes nanocrystalline TiO2 as the
photoabsorber
5
Solar Panel Cost
- Initially solar panels were expensive (gt2000 per
watt in 1950s). Thus their use was limited to
very special applications such as powering space
satellites. - Today solar panels are less than 4 per watt.
6
Whats on the Horizon?
First Generation Single and polycrystalline
wafer cells Second Generation Thin film
cells Third Generation Thin film cell efficiency
is increased by using multiple layers in tandem
and matching the band gaps of each layer to a
different region of the solar spectrum.
7
Evolution of the Efficiencyof the Steam Engine
8
Schematic of the Graetzel Cell
9
Useful Physics
- The adsorbed dye molecule absorbs a photon
forming an excited state. dye - The excited state of the dye can be thought of as
an electron-hole pair (exciton).
- The excited dye transfers an electron to the
semiconducting TiO2 (electron injection). This
separates the electron-hole pair leaving the hole
on the dye. dye - The hole is filled by an electron from an iodide
ion. 2dye 3I-? 2dye I3-
10
Useful Physics
- Electrons are collected from the TiO2 at the
cathode. - Anode is covered with carbon catalyst and injects
electrons into the cell regenerating the iodide.
- Redox mediator is iodide/triiodide (I-/I3-)
- The dashed line shows that some electrons are
transferred from the TiO2 to the triiodide and
generate iodide. This reaction is an internal
short circuit that decreases the efficiency of
the cell.
11
Key Step Charge Separation
- Charge must be rapidly separated to prevent back
reaction. - Dye sensitized solar cell, the excited dye
transfers an electron to the TiO2 and a hole to
the electrolyte. - In the PN junction in Si solar cell has a
built-in electric field that tears apart the
electron-hole pair formed when a photon is
absorbed in the junction.
12
Chemical Note
- Triiodide (I3-) is the brown ionic species that
forms when elemental iodine (I2) is dissolved in
water containing iodide (I-).
13
Construction Procedure
- TiO2 Suspension Preparation
- TiO2 Film Deposition
- Anthrocyanin Dye Preparation and TiO2 Staining
- Counter Electrode Carbon Coating
- Solar Cell Assembly
14
Preparing the TiO2 Suspension
- Begin with 6g colloidal Degussa P25 TiO2
- Incrementaly add 1mL nitric or acetic acid
solution (pH 3-4) nine times, while grinding in
mortar and pestle - Add the 1mL addition of dilute acid solution only
after previous mixing creates a uniform,
lump-free paste - Process takes about 30min and should be done in
ventilated hood - Let equilibrate at room temperature for 15 minutes
15
Deposition of the TiO2 Film
- Align two conductive glass plates, placing one
upside down while the one to be coated is right
side up - Tape 1 mm wide strip along edges of both plates
- Tape 4-5 mm strip along top of plate to be coated
- Uniformly apply TiO2 suspension to edge of plate
- 5 microliters per square centimeter
- Distribute TiO2 over plate surface with stirring
rod - Dry covered plate for 1 minute in covered petri
dish
16
Deposition of the TiO2 Film (cont.)
- Anneal TiO2 film on conductive glass
- Tube furnace at 450 oC
- 30 minutes
- Allow conductive glass to cool to room
temperature will take overnight - Store plate for later use
17
Examples TiO2 Plate
Good Coating Mostly even distribution
Bad Coating Patchy and irregular
The thicker the coating, the better the plate
will perform
18
Preparing the Anthrocyanin Dye
- Natural dye obtained from green chlorophyll
- Red anthocyanin dye
- Crush 5-6 blackberries, raspberries, etc. in 2 mL
deionized H2O and filter (can use paper towel and
squeeze filter)
19
Staining the TiO2 Film
- Soak TiO2 plate for 10 minutes in anthocyanin dye
- Insure no white TiO2 can be seen on either side
of glass, if it is, soak in dye for five more min - Wash film in H2O then ethanol or isopropanol
- Wipe away any residue with a kimwipe
- Dry and store in acidified (pH 3-4) deionized H2O
in closed dark-colored bottle if not used
immediately
20
Carbon Coating the Counter Electrode
- Apply light carbon film to second SnO2 coated
glass plate on conductive side - Soft pencil lead, graphite rod, or exposure to
candle flame - Can be performed while TiO2 electrode is being
stained
21
Assembling the Solar Cell
- Remove, rinse, and dry TiO2 plate from storage or
staining plate - Place TiO2 electrode face up on flat surface
- Position carbon-coated counter electrode on top
of TiO2 electrode - Conductive side of counter electrode should face
TiO2 film - Offset plates so all TiO2 is covered by
carbon-coated counter electrode - Uncoated 4-5 mm strip of each plate left exposed
22
Assembling the Solar Cell
- Place two binder clips on longer edges to hold
plates together (DO NOT clip too tight) - Place 2-3 drops of iodide electrolyte solution at
one edge of plates - Alternately open and close each side of solar
cell to draw electrolyte solution in and wet TiO2
film - Ensure all of stained area is contacted by
electrolyte - Remove excess electrolyte from exposed areas
- Fasten alligator clips to exposed sides of solar
cell
23
Measuring the Electrical Output
- To measure solar cell under sunlight, the cell
should be protected from UV exposure with a
polycarbonate cover - Attach the black (-) wire to the TiO2 coated
glass - Attach the red () wire to the counter electrode
- Measure open circuit voltage and short circuit
current with the multimeter. - For indoor measurements, can use halogen lamp
- Make sure light enters from the TiO2 side
light
Multimeter
solar cell
24
Measuring the Electrical Output
- Measure current-voltage using a 500 ohm
potentiometer - The center tap and one lead of the potentiometer
are both connected to the positive side of the
current - Connect one multimeter across the solar cell, and
one lead of another meter to the negative side
and the other lead to the load
25
Data Analysis
- Plot point-by-point current/voltage data pairs at
incremental resistance values, decrease
increments once line begins to curve - Plot open circuit voltage and short circuit
current values - Divide each output current by the measured
dimensions of stained area to obtain mA/cm2 - Determine power output and conversion efficiency
values
26
Results
- Current
- One solar cell 0.11 - 0.19 mA
- Two cells in parallel 0.164 - 0.278 mA
- Voltage
- One solar cell 0.30 0.40 V
- Resistance
- Very large.
Fig. 1 How many nano -physicists does it take
to screw in a lightbulb?
27
Questions
- What have we learned about the relationship of
solar cell to photosynthesis and solar energy? - How can you improve the procedure or design?
- How does this ultimately relate to other things
we've learned in NANOLAB?
28
Further Reading
- Konarka Technologies (Graetzel cells) http//www.k
onarkatech.com/ - PV Power Resource Site http//www.pvpower.com/
- US DOE Photovoltaics http//www.eere.energy.gov/pv
/ - Key Center for Photovoltaic Engineering http//www
.pv.unsw.edu.au/ - National Center for Photovoltaics http//www.nrel.
gov/ncpv/ - NRELs Photovoltaic Information Index http//www.nr
el.gov/ncpv/masterindex.html