Electrochemistry Oxidation and Reduction REDOX reactions PowerPoint PPT Presentation
1 / 61
Title: Electrochemistry Oxidation and Reduction REDOX reactions
1
Electrochemistry Oxidation and Reduction REDOX
reactions
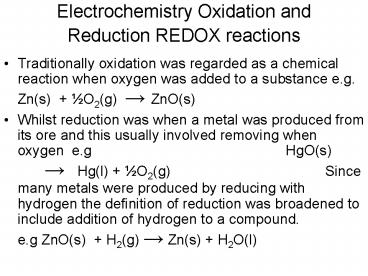
- Traditionally oxidation was regarded as a
chemical reaction when oxygen was added to a
substance e.g.
Zn(s)
½O2(g) ? ZnO(s) - Whilst reduction was when a metal was produced
from its ore and this usually involved removing
when oxygen e.g
HgO(s) ? Hg(l) ½O2(g)
Since many metals
were produced by reducing with hydrogen the
definition of reduction was broadened to include
addition of hydrogen to a compound.
e.g ZnO(s) H2(g) ? Zn(s) H2O(l)
2
- However it was soon recognised that there were
many similar reactions that did not involve
oxygen or hydrogen e.g. Zn(s)
S(s) ? ZnS(s)
and
2ZnS(s) C(g) ?
2Zn(s) CS2(l) - It did not make sense to make up new names for
each type of reaction and when the electronic
theory of matter was developed the basic unity
behind these reaction was seen.
3
Oxidation and electrons
- What is happening in both of the oxidation
reactions to the zinc is
Zn(s) ½O2(g) ?
ZnO(s) Zn(s)
S(s) ? ZnS(s)
Zn(s) ? Zn2 2 e-
i.e the zinc was losing two
electrons - and either an oxygen atom or a sulphur atom was
picking up two electrons from the zinc
O 2e- ? O2-
S 2e- ? S2-
4
Reduction and electrons
- In the case of the reduction the following was
happening. ZnO(s) H2(g) ? Zn(s)
H2O(l) 2ZnS(s) C(g) ? 2Zn(s)
CS2(l) Zn2 2e- ? Zn(s)
i.e. the zinc was gaining two electrons
5
Summarising
6
Oxidation can not occur without reduction
- You should have noticed in all these reaction
that as something is being reduced another
element or compound is being oxidised e.g
ZnO(s) H2(g) ? Zn(s) H2O(l)
The zinc is
being reduced (gaining electrons) but the
hydrogen atoms in the H2 molecule are losing
electrons - Zn2 2 e- ? Zn
- H2 ? 2H 2e-
- Because oxidation can not occur without reduction
taking place somewhere in the overall reaction we
call these reactions REDUCTION OXIDATION
reactions or
7
Half equations.
- We have seen that we can split these reactions
into half equations, one of which is the
oxidation
Zn(s) ? Zn2 2 e- (1) - And the other is that of the oxygen being reduced
O 2e- ? O2-
Since it
is oxygen molecule that is reacting we should
write O2 4e- ? 2O2- (2) - And by combining the two we get the overall
reaction. This is done by - (a) making sure we have the same number of
electrons involved in the oxidation as well as
the reduction
8
- We do this by multiplying equation 1 by 2 2Zn(s)
? 2Zn2 4 e- - Then we add the equations together
2Zn(s) O2 4e- ? 2Zn2 4e- 2O2- - We can simplify and add standard states
2Zn(s) O2(g) ? 2Zn2(s) 2O2- (s)
9
Oxidation Numbers and Oxidation states
- When we are looking at REDOX reactions it is
useful to introduce the concept of an oxidation
number or oxidation state. This tells us how
oxidised or reduced an element is in a compound
compared to its elemental state. So something
in a 3 state can be though of as having lost 3
electrons. However it is important to know that
the oxidation state of a compound does not tell
you anything about the bonding in a compound. It
is purely a bookkeeping tool. . So in many
compounds C is 4 e.g. in CCl4, but CCl4 does
not consist of ions it is a covalent liquid.
10
Oxidation numbers continued
11
Rules for assigning oxidation numbers
- Work through the following rules in the order
given. Stop as soon as the oxidation number has
been assigned -
Oxidation number - The sum of the oxidation
numbers of all
the atoms in the
species is equal to its
total charge - For all atoms in their elemental form 0
- For elements of Group 1 1
of Group 2
2
of Group 13 (except B) 3 for M3
1 for M
of Group 14 (except C and
Si) 4 for M4
2 for M2
12
Rules for assigning oxidation numbers( continued)
- 4 For hydrogen 1 in combination with
non- metals -1 in combination with
metals - 5 For Flourine -1 in all compounds
- For oxygen -2 unless combined with F
-1 in superoxides
(O22-)
½ in superoxides (O2-) - Cl,Br,I -1 unless combined with F or O
13
Examples
- Working out the oxidation state on an element is
not too difficult provided the rules are followed - What is oxidation state of S in SO2
- Nox(S) 2 x Nox(O) 0
- Nox(S) 2 x -2 0
- Nox(S) 4 0
- Nox(S) 4
14
Examples continued
- What is the oxidation state of Cl in ClO4-
- Nox(Cl) 4 x Nox(O) -1
- Nox(Cl) 4 x -2 -1
- Nox(Cl) -1 8
- Nox(Cl) 7
- What is the oxidation state of Cr in Cr2O7Na2
- 2Nox(Cr) 7 x Nox(O) 2Nox(Na) 0
- 2Nox(Cr) (7 x -2) (2 x 2) 0
- 2Nox(Cr) 14 2 0
- 2Nox(Cr) 12
- Nox(Cr) 6
15
Self test questions
- What is the oxidation state of the underlined
elements in each of the following compounds - MnO2, UF6 BrO3- Na2FeO4
16
Balancing equations (Acid conditions)
- Potassium nitrogen ? Chromium3
Potassium
dichromate monoxide
nitrate - K2Cr2O7 NO Cr3
KNO3 - Skeletal Equations
Cr2O72- ? Cr3
NO ? NO3- - Balance all elements except O H and e-
Cr2O72- ? 2Cr3
NO ? NO3- - Balance O and H
(a) add H2O to
balance O
Cr2O72- ? 2Cr3 7H2O
NO 2H2O ? NO3-
17
Balancing equations (Acid conditions)
- (a) add H to balance H
Cr2O72- 14H ?
2Cr3 7H2O NO 2H2O
? NO3- 4H - (b) Balance the charges by adding electrons
(1) Cr2O72- 14 H 6e- ?
2Cr3 7H2O (2) NO
2H2O ? NO3- 4H 3e-
18
Balancing equations (Acid conditions)
- Balance the number of electrons on both equations
and add
1 x
(1) 2 x (2)
Cr2O72- 14 H 6e-
2NO 4H2O ? 2Cr37H2O
2NO3- 8H 6e- - Simplify and cancel
Cr2O72- 6H 2NO ? 2Cr33H2O 2NO3-
- Put in standard states
Cr2O72- (aq) 6H(aq)
2NO(g) ? 2Cr3 (aq) 3H2O(l)
2NO3-(aq)
19
Balancing equations (Acid conditions)
- Of course the H ions are present as H3O ions
but for simplicities sake and convention they are
just represented as H
20
Balancing equations (Basic conditions)
- Potassium Bromide Manganese
Bromate permanganate Dioxide - 1 Skeletal l Equation
MnO4- ? MnO2
Br-
? BrO3- - Balance all elements except O H and e-
MnO4- ? MnO2
Br- ? BrO3- - Balance O and H (a) add H2O to balance O
MnO4- ? MnO2 2H2O
Br- 3H2O ? BrO3-
21
- Balancing equations basic conditions continued
- (b) Balance the H atoms by adding H2O to the side
needing H atoms and the same number of OH- to the
other side.
MnO4- 4H2O ? MnO2 2H2O
4OH- Br- 3H2O 6OH- ?
BrO3- 6H2O - (c) Add electrons to balance charge
(1) MnO4- 4H2O 3e- ? MnO2 2H2O
4OH- (2) Br- 3H2O
6OH- ? BrO3- 6H2O 6 e-
22
- Balancing equations basic conditions continued
- Balance the number of electrons on both sides 2 x
(1) (2) 2MnO4- 8H2O 6e- Br- 3H2O
6OH- ? 2MnO2 4H2O 8OH-
BrO3- 6H2O 6 e- - Simplify and cancel
2MnO4- H2O
Br- ? 2MnO2 2OH- BrO3- - Add standard states
2MnO4-(aq)H2O(l)Br-(aq)?
2MnO2(s)2OH- (aq) BrO3-(aq)
23
Self test questions
- Balance the following equations in acid solution,
given the following skeletal equations. - H3PO4 ? H3PO3
- IO3 - ? I2
- Balance the following equations in basic
solution, given the following skeletal equations.
- NO2 - ? NO
- SO4 2- ? SO32-
24
Oxidising and reducing reagents
- Cl2 is a powerful oxidising agent and Na is a
powerful reducing agent. The half equations for
the formation of NaCl being - Na (s) ? Na e-
Cl2 e- ? 2Cl-
2Na Cl2 ? 2NaCl
If
we look at the half equation we can se that the
chlorine (the oxidising agent) has been reduced
and Na the reducing agent has been oxidised, this
is perfectly general.
25
(No Transcript)
26
The electrochemical series.
- We know that gold does not rust, but what if it
was put in an atmosphere of fluorine what would
happen then. We are in fact able to predict what
would happen because of the electrochemical
series. This is a series of half reactions
arranged in the ordering of their reducing
ability. We are able to construct this series
because in a REDOX reaction electrons are
transferred.
27
Electrons produced by oxidation leave a
galvanic cell at the anode (?), travel through
the external circuit, and reenter the cell at the
cathode (?), where they cause reduction. The
circuit is completed inside the cell by migration
of ions through the salt bridge. A salt bridge is
unnecessary when the two electrodes share a
common electrolyte. (Taken from Atkins and Jones
without permission)
28
The reaction of CuSO4 and Zn
- In this cell we have the reaction of zinc metal
and copper sulphate - Zn CuSO4 ? ZnSO4 Cu
- Zn ? Zn2 2e-
- Cu2 2e- ? Cu(s)
- The electrode at which the copper which is being
reduced is called the CATHODE and is the positive
terminal. The electrode at which the zinc is
being oxidised is call the ANODE and is the
negative terminal In order to complete the
circuit the two solutions are connected via a
salt bridge which allows ions to flow through it
If we left this cell for long enough eventually
all the zinc anode would dissolve into solution.
29
This schematic picture of a galvanic cell
indicates the identities of the anode and
cathode, displays the oxidation and reduction
half-reactions, and shows the direction of
electron flow. (Taken from Jones and Atkins
without permission)
30
Reaction of copper metal and silver nitrate.
- If we had carried out the reaction using a silver
electrode instead of a zinc electrode a current
would again flow, but this time it would be the
silver that precipitated out and the copper
electrode that eventually all dissolved away. So
this time it would be the copper electrode would
be the anode and the silver electrode would be
the cathode
31
- So we can see that zinc will displace copper from
solution but that copper will displace silver.
Putting it another way we see that copper is a
better reducing agent than silver but that zinc
is better reducing agent than either copper or
silver.
32
Standard electrode potentials.
- What have done here is produce an electrochemical
cell. We can think that an electrode the
following situation occurring - M Mn ne-
- The better the reducing agent the more the
equilibrium lies over the right hand side.
33
Voltages and cells.
- If we had used a voltmeter in the circuit we
would find that the bigger voltage would be
developed for Zn/Ag, the second biggest by Zn /Cu
and the smallest by Cu/Ag. So we can see that
the voltage in a cell is governed the tendency of
the metal ions to go into solution represented by
the equilibrium we have shown above. So by
measuring the voltage (and the direction of flow
of the electrons) in a series of cells we will be
able to arrange oxidising and reducing agents in
an electrochemical series.
34
Figure 18.12 The cell potential can be thought
of as being the difference of the two reduction
potentials produced by the two electrodes. The
cell potential is positive if the cathode has a
higher potential than the anode. (Taken from
Jones and Atkins without permission)
- Ag
Cu
Zn
A
Ladder with an uneven tread
35
36
Figure 18.11The cell described in Self-Test
18.5A.
37
The standard hydrogen electrode.
- The standard electrode consists of a piece of
- platinum foil coated with fine particles of
platinum. - It is immersed into a solution of hydrogen ions
and - hydrogen gas is bubbled over it. Provided all
the - components are in their standard states, and the
- concentration of H(aq) ions is 1 mol dm-3 and
the - pressure is 1 atmosphere for the H2 gas the
standard - electrode potential Eº is define as 0 V
- (Of course in reality there are not H ions
present but H3O ions)
38
Other couples
- Electrode potentials can also be measured for
reactions of the type - Mn ? M(n1) e- such as
- Co2 ? Co3 e-
- To measure this potential we place a platinum
electrode into a solution which is both 1M in
Co2 and Co3 and connect it into a circuit.
39
Figure 18.13 The variation of standard
potentials in the main groups of the periodic
table. Note that the most negative values occur
in the s block and the most positive values occur
close to fluorine. Taken from Jones and Atkins
without pernission)
40
(No Transcript)
41
Points to note about standard electrode
potentials.
- The Eº values are also known a standard reduction
potentials In all the couples the oxidized
species is listed first e.g. (K(aq)/K(s) not
K(s)/K(aq) - Eº can be measured at different temperatures and
it does vary with temperatures except for
H(aq)H2 which is always defined to be 0.0 - Some values of E can not de determined directly
e.g . (K(aq)/K(s) but instead have to obtained
from other data. - The Eº really only applies to a couple so for .
(K(aq)/K(s) Eº means that in aqueous solution K
ions are formed not K- - The Eº values can be used to predict if a
reaction will go.
42
The cell diagram
- The is a convention to drawing an electrochemical
cell which is - reactant ? product reactant ? product
(-anode)
( cathode) (in which oxidation
(In which reduction
takes place)
takes place)
where the symbol denotes the salt bridge - Examples
- Pt H2(g) H(aq) Co3(aq) Co2(aq) Pt
- Zn(s) Zn2 (aq) Pt H(aq) H2(aq)(g)
43
Predicting if a reaction will go from Eº values.
- Write down both half-reactions. The reaction
which has the most negative (or least positive)
value should be placed at the top. - Draw anticlockwise arrows to predict if a
reaction will occur. - The ends of the arrows show what will be formed
(or left) at the end of the reaction (or lack of
reaction)
44
- Example
- Will H2 gas reduce Ce4 to Ce3
2H(aq) 2e- ?
H2(g) Eº 0.00 V
Ce4(aq) e- ? Ce3 Eº 1.61 V - Draw anticlockwise arrows
-
.
2H(aq) 2e- ? H2(g) Eº 0.00 V
Ce4(aq) e-
? Ce3 Eº 1.61 V
45
(No Transcript)
46
- Example 2
- Will I2 react with an aqueous solution of NaCl
- I2(aq) 2e- ? 2I-(aq) Eº 0.54
Cl2(aq) 2e- ? 2Cl-(aq) Eº 1.36 - .
47
Self test questions
- Using the standard reduction potentials in the
table given before say whether or not the
following reactions will occur - F2(aq) 2Br-(aq) ? Br2(aq) 2F-(aq)
Mg(s) 2Fe3(aq) ? Mg2(aq)
2Fe3(aq) Ag(s)
2H(aq) ? Ag(aq) H2(g) 2Fe3(aq)
2Cl-(aq) ? 2Fe2(aq) Cl2(aq)
48
(No Transcript)
49
(No Transcript)
50
The activity Series for metals
-
E /V - K(aq) e- ? K(s) -2.92 Ca2(aq)
2e- ? Ca(s) -2.87 Na(aq) e- ?
Na(s) -2.71 Mg2(aq) 2e- ? Mg(s) -2.37
Al3(aq) 3e- ? Al(s) -1.67 Zn2(aq)
2e- ? Zn(s) -0.76 Mn2(aq) 2e- ?
Mn(s) -1.18 Fe2(aq) 2e- ? Fe(s) -0.44
Ni2(aq) 2e- ? Ni(s) -0.25 Sn2(aq)
2e- ? Sn(s) -0.14 Pb2(aq) 2e- ?
Pb(s) -0.13 2H(aq) 2e- ? H2(s) 0.00
Cu2(aq) 2e- ? Cu(s) -0.34 Ag(aq) e-
? Ag(s) 0.80 Au(aq) e- ? Au(s) 1.68
51
The activity series of metals.
- This is a subset of the standard electrode
potentials. The metals are arranged in the order
of their reducing power. With the most reactive
metals at the top. A metal placed in solution
can displace a metal ion of a metal below it in
the reactivity series so - Zn(s) CuSO4(aq) ? ZnSO4(aq) Cu(s)
- Will proceed
52
Corrosion
- Many metals including iron corrode in air and
with water. We can predict which metal which
corrode with water by looking at the electrode
potentials. - Fe2(aq) 2e- ? Fe(s) E -0.44V
H2O(l) 2e- ? H2(g) 2OH-(aq) E -0.42
V - So water has a slight tendency to cause iron to
rust.
53
Iron nails stored in oxygen-free water (left) do
not rust because the oxidizing power of water
itself is weak. When oxygen is present (as a
result of air dissolving in the water, right),
oxidation is thermodynamically spontaneous and
rust soon forms. (Taken from Jones and Atkins
without permission)
54
The role of oxygen
- But when oxygen is present iron corrodes much
more rapidly. This is because if oxygen is
dissolved in water the following reduction
potential can apply - O2(g)4H(aq)4e- ? 2H2O (l) E 0.81 V
- Fe2(aq) 2e- ? Fe(s) E -0.44V
O2(g)4H(aq)4e- ? 2H2O (l)
E 0.81 V - So we can see in this reaction there is a much
greater difference in electrode potentials, so a
greater voltage for a cell and hence the reaction
will proceed.
55
Figure 18.22 The mechanism of rust formation.
(a) Oxidation of the iron occurs at a point out
of contact with the oxygen of the air, and the
surface of the metal acts as an anode in a tiny
galvanic cell. (b) Further oxidation of Fe2? to
Fe3? results in the deposition of rust on the
surface. (Taken form Jones and Atkins without
permission)
56
Rust the next step
- The Fe2 ions dissolves into solution where it is
further oxidised to produce Fe3 which the
deposits out of solution as hydrated iron oxide
Fe2O3 xH2O ( the x shows an indeterminate amount
of water). This rust is mechanically fragile and
can be easily dislodged. Because the Fe
dissolves first into solution no protective coat
of a passivating oxide as in Al2O3 is formed to
protect the surface. - Note the presence of dissolved ions such as Cl-
can speed up electrochemical reactions so that is
why iron objects rust faster near the seaside in
a salt water atmosphere.
57
Metals that rust but then are stable.
58
Prevention of rusting
- 1 Stop the air and water attacking the surface
by (a) painting, oiling, greasing, covering with
a plastic or by coating with a passivating oxide
e.g. tin in tinplating.
59
Metal girders are galvanized by immersion in a
bath of molten zinc. (Taken from Jones and Atkins
without permission).
- Cover with a metal with a more negative E so that
if the surface is scratched it will
preferentially dissolve away. This can be done
by coating with for example zinc (galvanizing) ,
Note that zinc itself forms a passivating zinc
oxide surface layer.
60
- 3 Sacrificial protection.
- Attach a more reactive metal e.g. Mg or Zn to
the object, which will preferentially dissolve
away.
61
Figure 18.24 In the cathodic protection of a
buried pipeline, or other large metal
construction, the artifact is connected to a
number of buried blocks of metal, such as
magnesium or zinc. The sacrificial anodes (the
magnesium block in this illustration) supply
electrons to the pipeline (the cathode of the
cell), thereby preserving it from oxidation.
(Taken from Jones and Atkins without permission)