Anthrax the sequel - PowerPoint PPT Presentation
1 / 27
Title:
Anthrax the sequel
Description:
Pleural fluid cell block from nonfatal case. Abundant Bacillus anthracis granular ... sponsors Intel and Microsoft, the project was completed in a stunning 24 days. ... – PowerPoint PPT presentation
Number of Views:68
Avg rating:3.0/5.0
Title: Anthrax the sequel
1
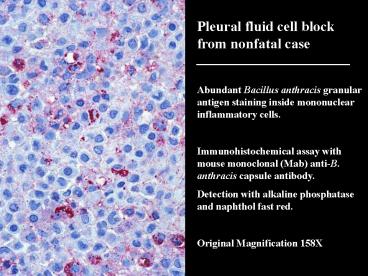
Pleural fluid cell block from nonfatal
case Abundant Bacillus anthracis granular
antigen staining inside mononuclear inflammatory
cells. Immunohistochemical assay with mouse
monoclonal (Mab) anti-B. anthracis capsule
antibody. Detection with alkaline phosphatase and
naphthol fast red. Original Magnification 158X
2
Mediastinal lymph node from a fatal
case Extensive capsular and sinusoidal
hemorrhage. Hematoxilin-Eosin stain. Origina
l Magnification 25X
3
Lymph node from same fatal case as the previous
slide Abundant B. anthracis granular antigen
inside mononuclear inflammatory cells . Some
bacilli (arrows) in the subcapsular hemorrhagic
area. Immunohistochemical assay with mouse
monoclonal (Mab) anti-B. anthracis cell wall
antibody. Detection with alkaline phosphatase and
naphthol fast red. Original Magnification 100X
4
Lung tissue from a fatal case B. anthracis
granular antigen staining inside a perihilar
macrophage (red arrow). Intra- and extracellular
bacilli (black arrow). Immunohistochemical assay
with mouse monoclonal (Mab) anti-B. anthracis
cell wall antibody. Detection with alkaline
phosphatase and naphthol fast red. Original
Magnification 100X
5
A closer look at the pathogenesis of Anthrax
McFadyean's reaction showing short chains of
Bacillus anthracis cells lying among amorphous,
disintegrated capsular material. White blood
cells can also be seen.
6
Virulence Factors of B. anthracis
pXO1
pXO2
pXO1 plasmid (110 MDa) codes for Anthrax toxin
(in 3 parts) PA Protective antigen 82.7 kDa -
Forms Heptamer EF Edema Factor 88.9 kDa -
Adenyl Cyclase LF Lethal Factor 90.2 kDa
Metalloprotease pXO2 plasmid (60 MDa) codes for
poly-D-glutamate capsule
7
Anthrax Vaccines
Anthrax Strains
Suspicion
NOTE A vaccine with NO virulence antigens
wouldnt work... ?
8
A medical illustrators ideal Bacillus
anthracis The poly-D-glutamate Capsule material
appears as a fluffy yellow cover of the bacillus
9
The acellular vaccine may need a little more
explanation...
- Virulent Bacillus anthracis, under appropriate
conditions - - at 37oC,
- - in the presence of serum factors,
- - with added CO2
- produce and secrete ANTHRAX TOXIN
- with its 3 components
- - PA, Protective Antigen
- - EF, Edema Factor
- - LF, Lethal Factor
10
AVA The standard anthrax vaccine in the United
States is approved by the Food and Drug
Administration and is routinely administered to
persons at risk for exposure to anthrax spores.
The existing supplies are currently being used to
immunize all military personnel. Designated
"anthrax vaccine adsorbed" (AVA), it is an
aluminum hydroxideprecipitated preparation of
protective antigen from attenuated,
nonencapsulated B. anthracis cultures of the
Sterne strain.
11
Anthrax Vaccine Adsorbed AVA, the only licensed
human anthrax vaccine in the United States, is
produced by BioPort Corporation in Lansing,
Michigan, and is prepared from a cell-free
filtrate of Bacillus anthracis culture that
contains no dead or live bacteria (60). The
strain used to prepare the vaccine is a
toxigenic, nonencapsulated strain known as
V770-NP1-R (50). The filtrate contains a mix of
cellular products including PA (57) and is
adsorbed to aluminum hydroxide (Amphogel, Wyeth
Laboratories) as adjuvant (49). The amount of PA
and other proteins per 0.5mL dose is unknown, and
all three toxin components (LF, EF, and PA) are
present in the product (57). The vaccine contains
no more that 0.83 mg aluminum per 0.5mL dose,
0.0025 benzethonium chloride as a preservative,
and 0.0037 formaldehyde as a stabilizer. The
potency and safety of the final product is
confirmed according to U.S. Food and Drug
Administration (FDA) regulations (61). Primary
vaccination consists of three subcutaneous
injections at 0, 2, and 4 weeks, and three
booster vaccinations at 6, 12, and 18 months. To
maintain immunity, the manufacturer recommends an
annual booster injection. The basis for the
schedule of vaccinations at 0, 2, and 4 weeks,
and 6, 12, and 18 months followed by annual
boosters is not well defined (52,62,63 Table 1).
12
(No Transcript)
13
(No Transcript)
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
(No Transcript)
18
(No Transcript)
19
(No Transcript)
20
Anthrax Pathogenesis Artistic representation from
the March 2002 SCIENTIFIC AMERICAN
feature Attacking Anthrax by John A.T. Young,
R. John Collier
PA
EF
LF
Scientific American 286 (3) 36-45
21
LF EF
Center Spread The ideas unfold
PA
22
1 PA Binds to Receptor ATR
2 PA is cleaved
Cell Membrane
3 Heptamer
Cytosol
4 EF LF bind
Endosome
5
5 Complex is endocytosed
6 pH causes heptamer to inject EF LF into
cytosol
LF
6
EF
23
The Treatment Ideas featured They are being
tested...
1
2
3
A 22-mer of a plug molecule worked 7,000
times better than the original monomer in
cell cultures and in rats
This idea works well in vitro It needs to be
scaled up
Each DNI molecule neutralizes 6 normal PA
molecules On top of that, DNIs are still
immunogenic!
The United Devices Anthrax Project worked on Idea
2 and has new promising candidate monomers
24
Anthrax Research Project Completed
On January 22, United Devices announced the
launch of the Anthrax Research Project. Prom
pted by recent events and a heightened concern
around the threat of anthrax, this project's goal
was to accelerate what is usually a
time-consuming step in the lengthy drug discovery
process. The project entailed presenting a key
protein component of anthrax into the general
rotation of the United Devices Member Community's
current virtual screening project, which works
with the MetaProcessor platform over the
Internet. This allowed UD Members to lend their
computers in the screening of 3.57 billion
molecules for suitability as a treatment for
advanced-stage Anthrax. http//members.ud.com/proj
ects/anthrax/
"The realm of life sciences is in for a radical
shift in its approach to drug discovery"Dr.
Graham Richards, Head of Computational
Chemistry,University of Oxford
United Devices is excited to announce that as of
February 14, 2002, the screening phase of the
Anthrax Research Project has been completed.
25
More on the United Devices Anthrax Project
Screening is only one step in a long drug
discovery process that ultimately must move from
the computational realm into the actual
laboratory. The project used a 5-time redundancy
rate for each molecule to ensure a high level of
accuracy and quality. With the invaluable help of
the UD Member Community, NFCR Centre for
Computational Drug Design in the Department of
Chemistry at the University of Oxford, and
corporate sponsors Intel and Microsoft, the
project was completed in a stunning 24 days. Dr.
Graham Richards, Chairman of the Chemistry
Department at Oxford and the Director of the
Centre for Computational Drug Design, called the
results "unprecedented," commenting, "Had we done
this using traditional methods, it would have
taken years instead of less than 4 weeks."
Preliminary indications are that we have
narrowed the original pool of 3.57 billion
molecules down considerably, having identified
over 300,000 crude unique hits in the course of
the project. This significantly reduces the next
phase of the discovery process, in which the
ranked hits will be further refined and analyzed,
accelerating the overall time to availability of
a treatment. Some Members of the UD Community
continued processing results over the weekend
while initial results were verified.
26
Host Cell
27
PA (Protective Antigen) Heptamer Top view
TARGET MOLECULE (gold)
TARGET MOLECULE (gold)
PA (Protective Antigen) Heptamer Side view