Graphics PowerPoint PPT Presentation
Title: Graphics
1
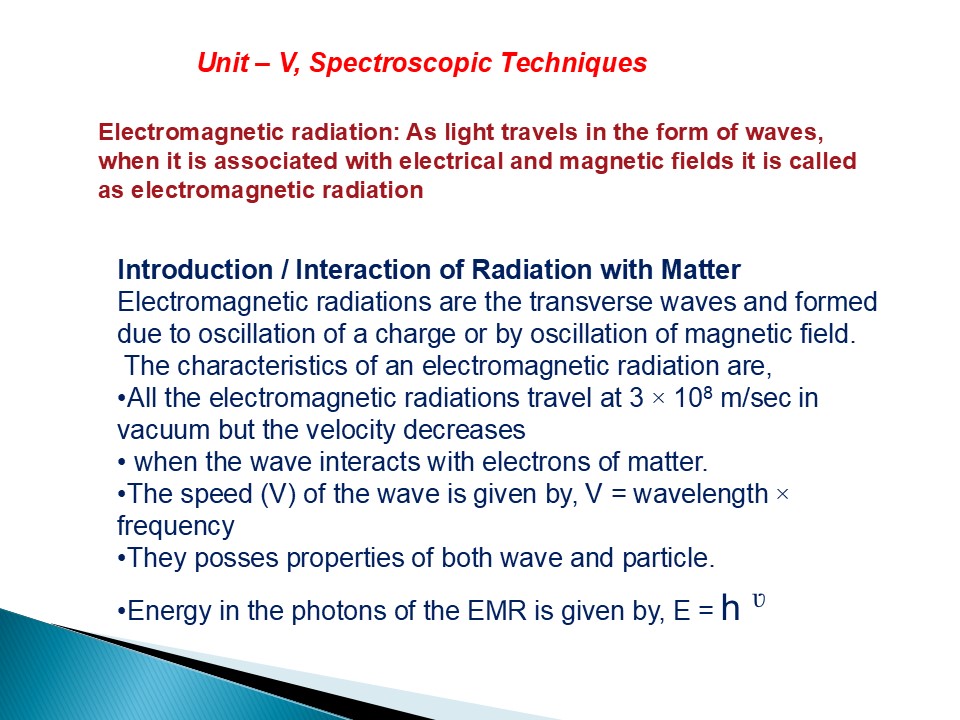
Unit V, Spectroscopic Techniques
Electromagnetic radiation As light travels in
the form of waves, when it is associated with
electrical and magnetic fields it is called as
electromagnetic radiation
- Introduction / Interaction of Radiation with
Matter - Electromagnetic radiations are the transverse
waves and formed due to oscillation of a charge
or by oscillation of magnetic field. - The characteristics of an electromagnetic
radiation are, - All the electromagnetic radiations travel at 3
108 m/sec in vacuum but the velocity decreases - when the wave interacts with electrons of
matter. - The speed (V) of the wave is given by, V
wavelength frequency - They posses properties of both wave and particle.
- Energy in the photons of the EMR is given by, E
h ?
2
Fundamentals of Spectroscopy Spectroscopy deals
with interaction of electromagnetic radiation
with matter. Due to these interactions ,energy is
absorbed or emitted by the matter. Measurement of
radiation frequency indicates the change in
energy involved. Spectroscopy involves
measurement of spectrum of a sample containing
atoms or molecules. Spectrum is a graph of
intensity of absorbed or emitted radiations by
sample versus frequency (v) or wavelength (?)
3
- The study of spectroscopy can be carried out
under following heads - Atomic spectroscopy It deals with interaction of
electromagnetic radiations with atoms. - Molecular spectroscopy It deals with interaction
of eletromangnetic radiations with molecules.
This results in transitions between rotational,
vibrational and electronic energy levels
4
- Spectroscopy is of two types
- spectroscopy and Emission spectroscopy
- Absorption spectroscopy In absorption
spectroscopy, a beam of polychromatic light falls
on a sample. Some part of light is absorbed by
the sample and remaining part is transmitted. The
intensity of transmitted light coming from sample
is measured at different wavelengths by suitable
photo detector. Then this information is
presented as a graph of intensity of absorbed
radiations. - Emission spectroscopy In this technique, sample
is subjected to intense source of energy like
electric arc. As a result, sample is vaporized
and electrons in the sample are excited to higher
energy state. When these electrons come back to
ground state energy level, they emit radiations.
These radiations are analysed by
spectrophotometer to generate spectrum that gives
information about atoms present in the sample.
5
- Like atoms, even molecules possess a set of
discrete energy levels, - which are of three types
- Electronic energy levels
- b) Vibrational energy levels
- c) Rotational energy levels
6
- Electronic energy levels At room temperature the
molecules are in the lowest energy levels E0.
When the molecules absorb UV-visible light from
electromagnetic radiation, one of the outermost
electrons from pi - bond, sigma bond or a lone
pair is promoted to higher electronic energy
state. - Vibrational energy levels Vibrational energy
levels are of less energy than electronic energy
levels. The spacing between vibrational energy
levels are relatively small. When infrared
radiation is absorbed molecules are excited from
one vibrational level to another. Thus, during
vibrational excitation molecule begins to
vibrate. - Rotational energy levels The spacing between
these levels is even smaller than vibrational
energy levels. The order of spacing of energy
levels is as follows. - ?E rotational lt ?E vibrational lt ?E electronic
7
Whenever electronic transition occurs by
absorption of UV radiation it is accompanied by
vibrational and rotational excitations.
Similarly, absorption of infrared radiation
leads to vibrational and rotational excitation.
8
Lamberts Law
The rate of decrease in intensity of radiation
is directly proportional to path length of
solution, when the monochromatic light passes
through a solution of constant concentration.
Where, I0 Intensity (radiant power) of the
incident radiation dx Small thickness of
solution or path length dI Small decrease in
intensity of light I0 It
9
Beers Law The rate of decrease in intensity
of light is proportional to concentration of
solution for a fixed thickness of solution
Combined Lambert-Beers Law
Lambert-Beers law can be stated as absorption
of a light by solution is directly proportional
to theconcentration of solution and the path
length
10
(No Transcript)
11
By taking unit concentration of sample in
solution (C 1 mole/lit) and unit length of path
(x 1 cm), the absorbance observed will be the
value of the constant in Lambert-beers equation.
A ? x C Î
is known as molar absorptivity, or molar
extinction coefficient constant. There is certain
wavelength which is absorbed maximum and it is
known as ?max. This wavelength is selected to
analyse the sample by spectrophotometers
12
- Principle and instrumentation involved in
UV-visible spectroscopy - Principle
- Following principles are involved in UV-visible
spectrophotometry - Beers law The absorbance of the monochromatic
light from UV-visible region is proportional to
concentration of the solution, for the constant
path length.
The absorption of ?max from UV-visible region
causes excitation of bonding electrons in a
covalent bond to higher energy antibonding
orbitals e.g. s s, p p, non-bonding
electrons (lone pair) to p etc.
13
Instrumentation in UV-Visible Spectrophotometry
(Spectrophotometer)
Light source (Source of radiation) The source of
radiation should provide sufficient intensity
over the wavelength region. It has high
intensity. The most common radiation sources used
are hydrogen discharge lamp, quartz halogen
lamps, deuterium gas discharge tube, mercury arc.
14
Monochromator A monochromator selects light of a
particular wavelength. It consists of entrance
slit, collimator, a grating or prism, exit slit.
A good monochromator provides very narrow
wavelength band. Glass prisms are used for work
in the visible region, quartz prisms are used for
UV-region
Sample cell It holds the sample either in the
form of solution or as such. Quartz cuvettes are
used in UV spectroscopy but for visible
spectroscopy glass cuvettes can be used.
Detector Transmitted light from sample cell
falls on the detector where it is converted into
electric current. It converts light energy
directly into electrical energy. The output
current of photo detector is directly
proportional to the intensity of light falling on
it. For UV spectroscopy Photoelectric cell,
photomultiplier tube, photovoltaic cell can be
used as photodetector Recorder It is a display
device, which automatically draws the spectrum.
The signal from monochromator is recorded
onx-axis while the absorbance of the sample is
displayed on y-axis. The absorption of light
(EMR) by a substance is governed by certain laws,
namely, Beer's law and Lambert's law
15
- Theory of Electronic Transitions Energy
absorbed from the ultraviolet visible region
causes excitation of electrons to higher energy
state. - s-electrons These electrons are involved in
saturated or sigma bonds. - p-electrons These electrons are involved in
unsaturated (double or triple bonds) compounds
and aromatics. - n-electrons These are the electrons of outermost
energy level (valence shell) of atom, which have
not participated in bonding. - They are also called as lone pair of electrons
and are present in p- orbitals. e.g. organic
compounds containing nitrogen, oxygen, sulphur,
halogens
16
- Types of Electronic Transitions -
4 allowed transitions s s n s ?? ??
n ??
2 non-allowed / forbidden transitions s ??
?? s Relative energies of these transitions
are
17
- I
- s ?s transition These transitions can occur in
compounds, in which all the electrons areinvolved
in single (sigma) bonds and there are no lone
pair of electrons on any atom in the molecule. - e.g. saturated hydrocarbons like ethane, methane.
As the energy required for the transition is very
large, the absorption band occurs in the far
ultraviolet region (100 to 135 nm) - n ? p transition These type of transitions are
shown by unsaturated molecules (containing
multiple bonds) which also contain atoms with
lone pair of electrons like oxygen, nitrogen,
halogen etc. Eg. C ?? , C ?? , ?? ??
show this transition. In aldehydes, ketones,
cyanides, -NO, etc the transition occurs due to
excitation of electron from p-orbital (lone pair
of electron) to antibonding p orbital.
18
- n ? s transition Saturated compounds with
atoms having lone pair of electrons can undergo
this transition, and the bands are observed in
the near UV region (150-225 nm). If this
transition is observed in the compound having p
p transition, then the ?max for this transition
shifts to the longer wavelength. - Eg. Alcohols (R-OH), amines (R-NH2), alkyl
halides (R-X) etc. - p ? p transition p electrons in a bonding
orbital is excited to corresponding antibonding
orbital p orbital . Compounds containing
multiple bonds like alkene, alkynes, carbonyl,
nitriles, aromatic compounds undergo this
transition. - Alkenes generally absorb in the region 170 - 205
nm and are difficult to record by
spectrophotometer. However, molecules containing
two or more conjugated double bonds absorb above
200 nm and can be easily recorded.
19
- .
- Terms in UV-Visible Spectrophotometry
- Chromophore The part of a molecule responsible
for imparting color, are called as chromospheres.
OR The functional groups containing multiple bond
and capable of absorbing radiations above 200 nm
due to n ? p p ? p transitions. e.g. NO2,
NO, CO, CN, CN, CC, CS, etc - Important points
- Conjugated double bonds if present in compound,
shifts the ?max to longer wavelengths. - Conjugation of CC- and carbonyl group shifts
the ?max to longer wavelengths eg. Ethylene, - acetone.
20
2. Auxochrome The functional groups attached to
a chromophore which modifies the ability of the
chromophore to absorb light, altering the
wavelength or intensity of absorption. OR The
functional group with non-bonding electrons that
does not absorb radiation in near UV region but
when attached to a chromophore alters the
wavelength intensity of absorption