Trodelvy 200 mg injection in india - PowerPoint PPT Presentation
Title:
Trodelvy 200 mg injection in india
Description:
The drug Sacituzumab govitecan-hziy is used to treat patients with unresectable locally advanced or metastatic triple-negative breast carcinoma and locally advanced or metastatic urothelial carcinoma by healthcare professionals. The active ingredient in the medicine, Sacituzumab govitecan-hziy, is a Trop-2-directed antibody and topoisomerase inhibitor conjugate. The recombinant monoclonal antibody (Sacituzumab govitecan-hziy) is produced by mammalian cells, while the small molecule components SN-38 and CL2A are produced via chemical synthesis. This medicine is supplied in 180 mg in single-use vials for intravenous infusion. Trodelvy 180 mg injection is readily available from several generic medicine suppliers. To know more about this drug, dial 1800-889-1064. – PowerPoint PPT presentation
Number of Views:21
Title: Trodelvy 200 mg injection in india
1
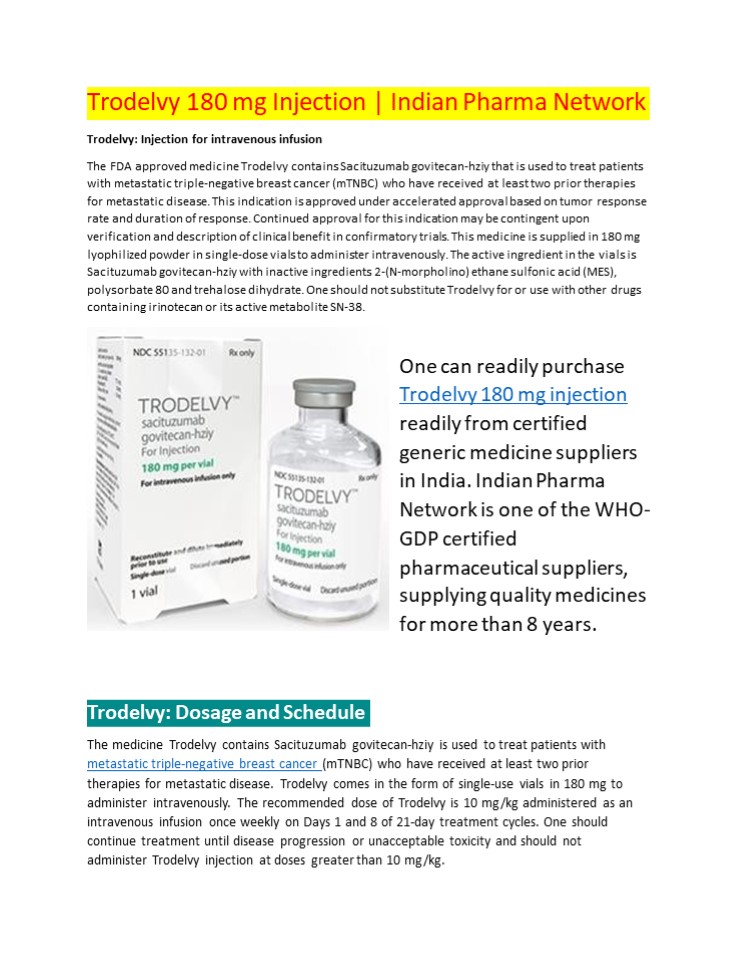
Trodelvy 180 mg Injection Indian Pharma Network
Trodelvy Injection for intravenous infusion The
FDA approved medicine Trodelvy contains
Sacituzumab govitecan-hziy that is used to treat
patients with metastatic triple-negative breast
cancer (mTNBC) who have received at least two
prior therapies for metastatic disease. This
indication is approved under accelerated approval
based on tumor response rate and duration of
response. Continued approval for this indication
may be contingent upon verification and
description of clinical benefit in confirmatory
trials. This medicine is supplied in 180 mg
lyophilized powder in single-dose vials to
administer intravenously. The active ingredient
in the vials is Sacituzumab govitecan-hziy with
inactive ingredients 2-(N-morpholino) ethane
sulfonic acid (MES), polysorbate 80 and
trehalose dihydrate. One should not substitute
Trodelvy for or use with other drugs containing
irinotecan or its active metabolite SN-38.
One can readily purchase Trodelvy 180 mg
injection readily from certified generic
medicine suppliers in India. Indian Pharma
Network is one of the WHO- GDP certified
pharmaceutical suppliers, supplying quality
medicines for more than 8 years.
Trodelvy Dosage and Schedule
The medicine Trodelvy contains Sacituzumab
govitecan-hziy is used to treat patients with
metastatic triple-negative breast cancer (mTNBC)
who have received at least two prior therapies
for metastatic disease. Trodelvy comes in the
form of single-use vials in 180 mg to administer
intravenously. The recommended dose of Trodelvy
is 10 mg/kg administered as an intravenous
infusion once weekly on Days 1 and 8 of 21-day
treatment cycles. One should continue treatment
until disease progression or unacceptable
toxicity and should not administer Trodelvy
injection at doses greater than 10 mg/kg.
2
One should administer Trodelvy as an intravenous
infusion only and should not administer as an
intravenous push or bolus. In the first infusion
one should administer infusion over 3 hours.
Observe patients during the infusion and for at
least 30 minutes following the initial dose, for
signs or symptoms of infusion-related reactions.
In case of subsequent infusions one should
administer infusion over 1 to 2 hours if prior
infusions were tolerated and observe patients
during the infusion and for at least 30 minutes
after infusion. Healthcare professionals
recommend prior to each dose of Sacituzumab
govitecan 180 mg, premedication for prevention
of infusion reactions and prevention of
chemotherapy induced nausea and vomiting
(CINV). TRODELVY FDA APPROVAL- FDA grants
regular approval to sacituzumab govitecan for
triple-negative breast cancer Source -
https//helpforheallth.blogspot.com/2022/11/trodel
vy-180-mg-injection-price-in- india.html