Efflux pumps in hepatocytes: - PowerPoint PPT Presentation
1 / 16
Title:
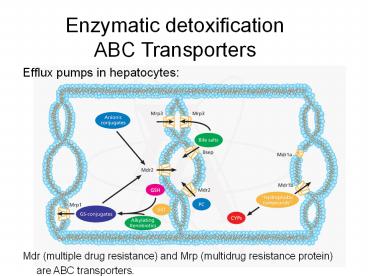
Efflux pumps in hepatocytes:
Description:
The hydrophobicity of various peptides of the transporter can be tested with Trp ... Example for the hydrophobicity test with Trp as a fluorescent probe. ... – PowerPoint PPT presentation
Number of Views:243
Avg rating:3.0/5.0
Title: Efflux pumps in hepatocytes:
1
- Efflux pumps in hepatocytes
Mdr (multiple drug resistance) and Mrp (multidrug
resistance protein) are ABC transporters.
2
- Drug pumps
- 3 superfamilies of proton-driven antiporters
(dominant in prokaryotes) - SMR (small multidrug resistance) trimer with 4
helices each, - MF (major facilitator) 12 helices and large
cytoplasmic loop (tetracycline transporter) - RND (resistance-nodulation-cell division) 12
helices and 2 large cytoplasmic loops - 1 superfamily of ATP-driven transporters
(dominant in eukaryotes) - ABC (ATP-binding cassette) 2x6 helices, 2
cytoplasmic ATP-binding cassettes
3
- The RND transporters
- TolC AcrAB pump of E. coli
- Cytosolic and periplasmatic antibiotics can be
pumped out into the medium.
4
- Drug pumps Quaternary structure of ABC
transporters
5
- Multi-drug resistance related protein structure
- Complete ABC transporter structures have only
recently be solved. The structure of the
membrane-bound part can be deduced from secondary
structure prediction - For each amino acid ten residues on both sides
are taken and their hydrophobicity averaged. Long
stretches of hydrophobic positions are predicted
to be transmembrane helices.
6
- Multi-drug resistance related protein structure
prediction of transmembrane helices for human
P-glycoprotein by the TMHMM server (DTU,
Denmark) - 10 of 12 helices identified.
7
- Multi-drug resistance related protein structure
- Prediction of transmembrane helices for human
multidrug-resistance protein - 16 of 17 helices identified. C terminus is
predicted outside because of only one missing
helix!
8
- Multi-drug resistance related protein
9
- Multi-drug resistance related protein
- MRP has the typical (archetypal) elements for an
ABC protein (and more) - TM-NBD-TM-NBD TM six helix transmembrane-domain
, NBD nucleotide binding domain - In most cases the nucleotide binding sites are
close to each other and the ATPase activity is
stimulated by substrate binding.
10
- The arsenical pump ArsA/ArsB
- ArsA ATPase with two ATP-binding sites and
AsO2--binding site - ArsB membrane translocase
- The binding site for AsO2- in ArsA is composed of
two Cys in one and one Cys in the other domain.
Upon binding of AsO2-, the ATP-binding sites come
together and are stimulated. - The coupling of ATP hydrolysis and AsO2- (or the
drug in general) transport is not understood.
11
- The arsenical pump ArsA/ArsB
- The hydrophobicity of various peptides of the
transporter can be tested with Trp as a
fluorescent probe.
Hydrophilic environment Weaker
fluorescence, Short wavelength emission
Hydrophobic environment Stronger
fluorescence, Long wavelength emission
Trp
Trp
12
- Example for the hydrophobicity test with Trp as a
fluorescent probe.
Fluorescence emission of native and denatured
asparaginase
13
- The arsenical pump ArsA/ArsB
- Conformational changes in ArsA between ATP and
ADP complexes. The N-terminal end of the marked
consensus sequence seems to be in hydrophilic
environment upon ATP-binding and the C-terminal
end hydrophobic. With ADP its the other way
around. This single-turnover event may provide
the power-stroke for the translocation of
arsenite across the membrane
...DTAPTGHTIRLL...
14
- Structural information on ArsA
- X-ray and EM structures of ArsA have been
obtained. The EM shows trimers of ArsA arranged
in a circle. - The X-ray structure shows the connection between
the metal-binding site and the ATP-binding site.
Single particles
2D crystal
15
- Structural information on ArsA
After metalloids (As, Sb) bind, the blue peptide
changes its conformation and activates ATPase
activity. This stimulates arsenite translocation
across the membrane. The conformational changes
are not known.
16
- Drug resistance in Candida albicans
- Strains resistant to the normally successful
azole (imidazole-, triazole-derivatives) drugs
have developed three major resistance mechanisms
- bypass mutations in antifungal target genes
- upregulation of Erg1, the cytochrome P450
lanosterol demethylase, which is the main azole
target - increased drug efflux by overexpression of drug
efflux pumps Cdr1p and Cdr2p (ABC transporters)