NOVEL DRUG DELIVERY SYSTEMS PowerPoint PPT Presentation
Title: NOVEL DRUG DELIVERY SYSTEMS
1
NOVEL DRUG DELIVERY SYSTEMS
2
Introduction
- Transdermal drug delivery systems These are
defined as self-contained, discrete dosage forms
which when applied to the intact skin, deliver
the drug(s) through the skin at a controlled rate
to the systemic circulation. - Advantages
- Transdermal medication delivers a steady infusion
of a drug over an extended period of time.
Adverse effects or therapeutic failures
frequently associated with intermittent dosing
can also be avoided. - Transdermal delivery can increase therapeutic
value of many drugs by avoiding specific problems
associated with the drug (ex- GIT irritation, low
absorption, decomposition due to hepatic first
pass effect, formation of metabolites that causes
side effects, short half life necessitating
frequent dosing etc). - Self administration is possible with these
systems. - The drug input can be terminated at any point of
time by removing transdermal patch.
3
Transdermal drug delivery systems
- Dis advantages
- The drug must have some desirable physicochemical
properties for penetration through stratum conium
if the drug dosage required for therapeutic
value is more than 10mg/day, the transdermal
delivery will be difficult for administration.
Daily doses of less than 5mg/day is preferred. - Skin irritation or contact dermatitis due to
drug, excipients and enhancers of drug used to
increase percutaneous absorption is another
limitation. - Clinical need is another area that has to be
examined carefully before a decision is made to
develop a transdermal product. - The barrier function of skin changes from one
site to another on the same person, from person
to person with age.
4
Kinetics of transdermal penetration
- Knowledge of skin permeation kinetics is vital to
the successful development of transdermal
therapeutic systems. Transdermal permeation of
drug involves the following steps - Sorption by stratum corneum
- Penetration of drug through viable epidermis
- Uptake of the drug by capillary network in the
dermal papillary layer - Basic components of transdermal drug delivery
systems - 1) Polymer matrix/matrices Natural polymers
Cellulose, gelatin, starches. - b) Synthetic elastomers polybutadiene,
polysiloxane, neoprene etc. - c) Synthetic polymers PVC, PVA, polyethylene,
polyurea. - 2) Drugs
- 3) Permeation enhancers These are compound
which promote skin permeability by altering the
skin as a barrier to the flux of a desired
penetrant. A large no of compounds are identified
as solvents, surfactants, binary systems
miscellanous chemicals. - 4) Other excipients It includes adhesives,
backing membrane.
5
Approaches used in development of transdermal
systems
- Membrane permeation controlled systems
- Nitroglycerin releasing transdermal system,
clonidine releasing transdermal system. - Adhesive dispersion systems
- Isosorbide dinitrate releasing transdermal system
- Matrix diffusion controlled
- Nitro-Dur-I, Nitro-Dur-II
- Microreservoir / Microsealed dissolution
controlled systems - Nitrodisc (nitroglycerine releasing transdermal
systems).
6
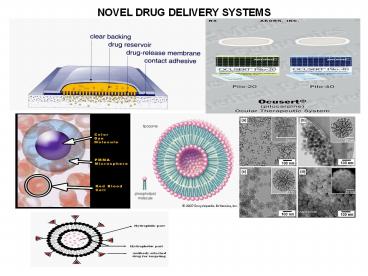
Ocular drug delivery systems
- The objective of the ocular drug delivery is to
- Improve ocular contact time
- Enhancing corneal permeability
- Enhancing site specificity
- Role of polymers in ocular drug delivery
- Incorporation of polymers into an aqueous medium
of drug could increase solution viscosity and
reduces the solution drainage. Increasing the
solution viscosity of pilocarpine from 1 to 100
cps by using methyl cellulose reduced the drug
drainage and 2 fold increase in drug
concentration in aqueous humor was obtained. - Natural polymers such as sodium hyaluronate
chondroitin sulfate are being investigated as
viscosity inducing agents. - Ophthalmic inserts It offers the potential
advantage of improving patient compliance by
reducing dosing frequency. The desired criteria
for a controlled release ocular insert are - a) Comfort b) Ease of handling c) sterility d)
Ease of manufacture. - Controlled release systems for ocular use
encompass both erodible non-erodible systems.
The non-erodible inserts are of 2 types - Ocusert system
- Contact lens
7
Ocular drug delivery systems
- Ocusert systems It is preprogrammed to release
pilocarpine at constant rate of 20 or 40 µg/hr
around the clock for 7 days for the treatment of
glaucoma. - Contact Lens Therapeutic soft lenses are often
used to aid corneal wound healing in patients
with infection, corneal ulcers characterised by
thining of cornea. - Erodible Inserts Several erodible drug inserts
have been prepared tested for ocular use.
Pilocarpine containing CMC wafers, PVA disc or
rod is a classical example. The three devices of
erodible inserts have been marketed to date are
a) The Lacriserts b) SODI (soluble ocular drug
insert) c) Minidisc. - Corneal collagen shields Collagen is a protein
that can be safely applied to the body and is
used to promote wound healing and delivers a
variety of medications to the cornea ocular
tissues. A study published in 1978 showed that
wafer shaped collagen inserts impregnated with
gentamicin produced highest level of drug in tear
film tissue in the rabbit eye compared to
drops, ointment conjuctival injection.
8
Buccal drug delivery systems
- Drugs administered to the oral cavity are removed
from the site of administration by natural
clearance mechanisms. - For drug delivery purposes, the term bioadhesion
implies attachment of a drug carrier system at a
specific biological action. In most instances the
bioadhesive polymer is in contact with the mucous
hence the term mucoadhesion is employed. - Buccal mucosa The buccal cavity provides a
highly vascular mucous membrane site for
administration of drugs. - The epithelial lining of oral cavity differs both
in type (keratinised non-keratinised)
thickness in different areas the differences
give rise to regional variations in permeability
to drugs. - The buccal mucosa is being perceived as an
alternative for peptide protein drug
administration especially when sustained delivery
is desired. - The future challenge in the development of
buccoadhesive dosage forms is to modify the
permeability barrier of the mucosa using safe and
effective penetration enhancers.
9
Buccal drug delivery systems
- Mucoadhesive buccal dosage forms have 3 desirable
features. - They are readily localised in the oral cavity to
improve enhance the bioavailability of drugs. - They facilitate intimate contact of the
formulation with the underlying absorption
surface. - They also prolong residence time of the dosage
form to permit once or twice a day dosing. - Methods to study bioadhesion
- Study of cellular modifications during
interpretation - Study of adhesion on artificial media
- Study of adhesion on biological tissues
- Factors affecting bioadhesion
- The bioadhesive polymer environment both affect
bioadhesion. The polymer related factors include
mol.wt, polymer chain length configuration,
concentration of active polymer swelling. - The environment related factors are pH applied
strength.
10
Liposomes as drug carriers
- These are simple microscopic (lipid) vesicles in
which an aqueous volume is entirely enclosed by a
membrane composed of lipid molecule. - The drug molecules can either be encapsulated in
aqueous space or intercalated into the lipid
bilayer. - Amphipathic molecules are used to form Liposomes.
Some examples of amphipathic molecules are
lecithin, phosphatidyl glycerol etc. - The exact location of drug will depend upon its
physicochemical characteristics the
composition of lipids. - A standard composition of Liposome is egg
lecithin cholesterol phosphatidyl glycerol in
molar ratio (0.9 1.0 0.1). These lipids can
be stored either as solids, or inorganic solution
at -20 or -70ºC in order to reduce the chances of
oxidation. - Method of preparation of Liposomes It involves
3 or 4 basic stages - Drying down lipids from organic solvent
- Dispersion of lipids in aqueous media
- Purification of resultant Liposomes
- Analysis of final product
11
Liposomes as drug carriers
- Characterisation of Liposomes
- The behaviour of Liposomes in both physical
biological systems is governed by the factors
such as physical size, membrane permeability,
entrapped solutes, chemical composition as well
as quantity purity of starting materials. - Therefore the Liposomes are characterised for
physical attributes (shape, size its
distribution, drug captured, entrapped volume,
lamellarity, drug release and chemical
composition (estimation of phospholipids,
cholesterol). - Applications of Liposomes
- Liposomes prove to be efficient carrier for
targeting the drug to site of action, because of
being biodegradable identical to biological
membrane. - Liposomes can able to produce localised drug
effect, enhanced drug uptake cell Liposome
interaction. - Liposomes are carriers for vaccines, antigens,
micromolecules for site specific delivery (oral,
topical, pulmonary ophthalmic etc).
12
Niosomes as drug carriers
- Niosomes are non-ionic surfactant vesicles that
can entrap both hydrophilic and lipophilic drugs
either in aqueous layer or in vesicular membrane
made of lipid materials. Niosomes can prolong the
circulation of entrapped drugs. - Some examples of non-ionic surfactants like
span-40,60,80 tweens are commonly used. These
surfactants can be combined with cholesterol to
entrap drugs in vesicles. - Formulation of Niosomes It can be formulated by
lipid layer hydration method, reverse phase
evaporation techniques or by trans membrane pH
gradient uptake process. - Characterisation Niosomes can be characterised
by size distribution studies (small niosomes
100-200 nm, large 800-900 nm, big 2-4 µm). - Evaluation Drug entrapment efficiency, drug
stability, drug leakage in saline plasma on
storage, PK aspects, toxicity studies drug
targeting efficiency.
13
Niosomes as drug carriers
- Loading of drug(s) into Niosomes The use of
niosomes as drug delivery vehicles naturally
assumes an ability to efficiently load the
niosomes with the drug of choice. Passive
trapping and active trapping are 2 methods used
to load drug(s) into niosomes. - Benefits of Niosomal drug carrier
- Niosomes are more suitable for parenteral drug
delivery. - As compared to liposomes, about 50 of
phospholipids can be replaced with non-ionic
surfactant the vesicle stability may be
improved. - Due to presence of non-ionic surfactants, there
may be improvement in permeation release of
drugs entrapped through various barriers of body
organs which may improve the targeting
efficiency of drugs. - The drug targeting efficiency of niosomes may be
improved using suitable surface modification with
the help of other adjuvants.
14
Niosomes as drug carriers
- Applications of Niosomes
- Therapeutic agents like anticancer agents, anti
infectives, anti HIV agents, antivirals,
anti-inflammatory drugs can be entrapped in
niosomes to achieve better bioavailability
targeting properties for reducing the toxicity
side effects of drugs. - Niosomes can be transported by macrophages which
are known to infiltrate tumour cells.
15
Microspheres as drug carriers
- Microspheres of biodegradable non biodegradable
polymers have been investigated for sustained
release depending on the final application. - The most important characteristic of microsphere
is microphase separation morphology which endows
it with a controllable variability in degradation
rate also drug release. - Preparation of Microspheres The preparation of
microspheres from natural polymers involves 3
steps - In the 1st step, the solution of polymer is
dispersed in a continuous medium such as
vegetable oil or an organic solvent using
suitable stabilising agent. - Dispersion is accomplished using
mechanical stirring or by ultrasonication or by
high speed homogenisation depending on particle
size required. - The 2nd step involves hardening of polymer
droplets either by heat denaturation or by
chemical cross linking using suitable cross
linking agent. - The 3rd step involves separation of solid
microspheres, purification drying.
16
Microspheres as drug carriers
- Drugs are incorporated into the microspheres
either during their synthesis or after the
microsphere is formed. - High loading can be achieved by insitu loading
if drug is insoluble in the dispersion medium
employed for microsphere stabilisation. - Washing the microspheres after their preparation
to remove surfactants, oils and other impurities
etc using solvents in which the drug solubility
is high may result in poor loading efficiency. - Mechanism of drug release from microspheres
- Degradation controlled monolithic system
- Diffusion controlled monolithic system
- Diffusion controlled reservoir system
- Erodable polyagent system
17
Microspheres as drug carriers
- Microspheres based on natural polymers
- Albumin microspheres
- Casein microspheres
- Gelatin microspheres
- Polysaccharide microspheres
- Microspheres based on synthetic polymers
- Polyester microspheres
- Polyanhydride microspheres
- Other biodegradable polymers
18
Nanoparticles as drug carrier
- Nanoparticles are sub nanosized colloidal
structures composed of synthetic or semisynthetic
polymers. - The first reported nanoparticles were based on
non biodegradable polymeric systems
(polyacrylamide, polymethyl methacrylate
polystyrene etc). - The polymeric nanoparticles can carry drug(s) or
proteinaceous substances (antigens). The drugs
may be added during preparation of nanoparticles
or to the previously prepared nanoparticles. - TYPES OF POLYMERS FOR PREPARATION OF
NANOPARTICLES - Natural polymers Lectins, albumin, alginate,
dextran, chitosan etc. - Synthetic polymers poly lactic acid, poly
lactide co glycolide, polymethyl methacrylate,
polybutyl cyanoacrylate.
19
Nanoparticles
- Preparation of Nanoparticles
- Amphiphilic macromolecule cross linking
- a) Heat cross linking b) Chemical cross
linking - 2) Polymerisation based methods
- Polymerisation of monomers insitu
- Emulsion (micellar) polymerisation
- Dispersion polymerisation
- Interfacial condensation
- Interfacial complexation
- 3) Polymer precipitation methods
- Solvent extraction/evaporation
- Solvent displacement (nanoprecipitation)
- Salting out
20
Nanoparticles
- Novel nanoparticulate systems
- Solid Lipid Nanoparticles (SLN), Copolymerised
Peptide Nanoparticles - Hydrogel nanoparticles, Nanocrystals
Nanosuspensions - Biomimetic nanoparticles , Magnetic nanoparticles
- Nanoparticles coated with antibodies
- Characterisation of nanoparticles
- Particle size size distribution
- Charge determination, surface hydrophobicity
- Chemical analysis of surface, carrier-drug
interaction, drug stability - Release profile, nanoparticle dispersion
stability - Applications of Nanoparticles
- 1) Cancer chemotherapy 4) DNA delivery
- 2) Ocular delivery 5)
Oligonucleotide delivery - 3) Brain delivery 6)
Lymph targeting