Transition metal organometallic compounds & Catalysis - PowerPoint PPT Presentation
1 / 41
Title:
Transition metal organometallic compounds & Catalysis
Description:
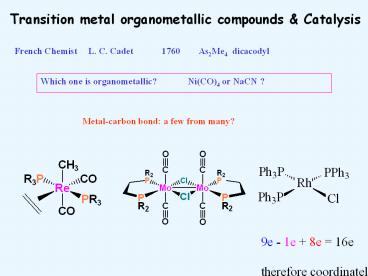
Transition metal organometallic compounds & Catalysis French Chemist L. C. Cadet 1760 As2Me4 dicacodyl Which one is organometallic? Ni(CO)4 or NaCN ? – PowerPoint PPT presentation
Number of Views:1747
Avg rating:3.0/5.0
Title: Transition metal organometallic compounds & Catalysis
1
Transition metal organometallic compounds
Catalysis
French Chemist L. C. Cadet 1760 As2Me4 dicacodyl
Which one is organometallic? Ni(CO)4 or NaCN ?
Metal-carbon bond a few from many?
2
Organometallic Compound Looking closer
3
18 en rule
1920 British Chemist Sidgwick
Organic compounds Octet rule
Organometallic 18 electron rule
18 valance electron inert gas configuration
4
(No Transcript)
5
Counting the number of electrons
To determine the electron count for a metal
complex Determine the oxidation state of the
transition metal center(s) and the metal centers
resulting d-electron count. To do this one
must a) note any overall charge on the metal
complex b) know the charges of the ligands
bound to the metal center c) know the
number of electrons being donated to the metal
center from each ligand 2) Add up the
electron counts for the metal center and ligands
6
Counting the number of electrons
7
(No Transcript)
8
(No Transcript)
9
Catalysis
A catalyst lowers the activation barrier for a
transformation, by introducing a new reaction
pathway.
It does not change the thermodynamics!!
Heterogeneous
Homogeneous
10
Catalysis Why?
Synthesis of chemicals pharmaceutical,
agricultural
Catalytic converter environmental
Biological system efficient catalyst
Organometallic compounds, metals etc.
11
How to select an efficient catalyst?
Activity related to rate of reaction (also
called turnover) efficient catalyst good
activity Turnover frequency (N) N
?/Q Large turnover frequency efficient
catalyst Selectivity Byproducts should be
minimized Lifetime It is costly to replace the
catalyst frequently Cost The acceptable cost
depends upon the catalyst lifetime, product value
lifetime and product value Poisoning
decomposition of catalyst, adsorption of
reactant/product
12
Coordination compounds in catalysis Nobel Prizes
- Yves Chauvin,Robert H. Grubbs
- and Richard R. Schrock.
- 2001 KNOWLES, NOYORI, SHARPLESS
- 1973 WILKINSON
- 1963 ZIEGLER, NATTA
- 1918 HABER
- 1909 OSTWALD
13
Hydrogenation of Unsaturated Hydrocarbons
NOBEL 2001
The most common catalyst ? Wilkinsons Catalyst,
RhCl(PPh3)3
14
Wilkinsons Catalyst (WC)
Chlorotris(triphenylphosphine)rhodium(I)
square planar d8 configuration
15
Geoffrey Wilkinson
- Born July 14, 1921, Yorkshire, England
- Ph.D from Cal Berkeley studying with Glenn
Seaborg - First published compound in 1965 in Journal of
the Chemical Society - Chemical Communications - Nobel Prize in Chemistry 1973 (shared with Ernst
Otto Fischer) for their pioneering work,
performed independently, on the chemistry of the
organometallic, so called sandwich compounds.
Organometallic compounds prepared by Wilkinson in
display at Harvard Univ.
16
Synthesis of WC
Commercially available
17
Catalytic steps
(a) Ligand coordination and dissociation
Facile coordination of the reactant and facile
loss of products.
Coordinatively unsaturated - 16-electron
complexes
(b) Oxidative addition
-occurs when a complex behaves simultaneously as
a Lewis base and a Lewis acid
Metal must possess a non-bonding electron pair
Coordinatively unsaturated
Oxidation of metal by two units Mn to Mn2
18
Oxidative addition
19
(c) Insertion or migration
Migration of alkyl and hydride ligands
20
(No Transcript)
21
(d) Nucleophilic attack
22
(d) Reductive elimination
Involves decrease in the oxidation and
coordination number
23
Hydrogenation of Unsaturated Hydrocarbons
?G0 -101 kJ/mol
24
WC in alkene Hydrogenation Catalytic Steps
(1) Oxidative addition
(2) Ligand Dissociation
25
WC in alkene Hydrogenation Catalytic Steps
(3) Ligand Association
(4) Migration/Insertion
26
WC in alkene Hydrogenation Catalytic Steps
(5) Ligand association
27
WC in alkene Hydrogenation Catalytic Steps
(6) Reductive elimination
28
WC IN A C T I O N
29
WC in alkene Hydrogenation Additional Notes
Rate of the reaction decreases as the alkyl
substitution increases Highly sensitive to the
nature of the phosphine ligand Analogous
complexes with alkylphosphine ligands are
inactive Highly selective for CC over CO
Applications
Laboratory scale organic synthesis Production
of fine chemicals
30
Alkene Hydrogenation Chirality Nobel
Chiral phosphine ligands have been developed to
synthesize optically active products. Synthesis
of L-DOPA (Used in the treatment of Parkinsons
diseases) Synthetic route was developed by
Knowles co-workers at Monsanto
Dr. William S. Knowles received Nobel prize in
chemistry 2001 along with other two scientists.
31
Alkene Hydrogenation, Chirality Nobel
32
(No Transcript)
33
Non-superimposable mirror image
Enantiomeric excess (moles of major enantiomer
- moles of other enantiomer / Total moles of both
enantiomers) 100
34
phenylanisylmethylphosphine (PAMP)
Dimeric product is DiPAMP
35
(No Transcript)
36
Additional notes For interested students
(a) Ligand coordination and dissociation
Facile coordination of the reactant and facile
loss of products.
Coordinatively unsaturated - 16-electron
complexes
(b) Oxidative addition
-occurs when a complex behaves simultaneously as
a Lewis base and a Lewis acid
Metal must possess a non-bonding electron pair
Coordinatively unsaturated
Oxidation of metal by two units Mn to Mn2
37
Oxidative addition
38
(c) Insertion or migration
Migration of alkyl and hydride ligands
39
(No Transcript)
40
(d) Nucleophilic attack
41
(e) Reductive elimination
Involves decrease in the oxidation and
coordination number