AP Physics Review Ch 12-15 PowerPoint PPT Presentation
Title: AP Physics Review Ch 12-15
1
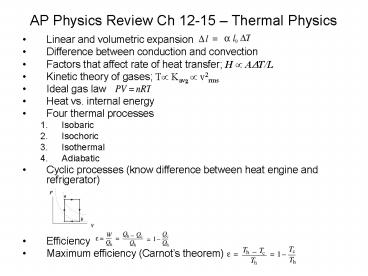
AP Physics Review Ch 12-15 Thermal Physics
- Linear and volumetric expansion
- Difference between conduction and convection
- Factors that affect rate of heat transfer H ?
A?T/L - Kinetic theory of gases T? Kavg ? v2rms
- Ideal gas law
- Heat vs. internal energy
- Four thermal processes
- Isobaric
- Isochoric
- Isothermal
- Adiabatic
- Cyclic processes (know difference between heat
engine and refrigerator) - Efficiency
- Maximum efficiency (Carnots theorem)
2
Which system has the largest average
translational kinetic energy?
- 1 mol of He at p 1 atm, T 300 K
- 2 mol of N2 at p 0.5 atm, T 450 K
- 2 mol of He at p 2 atm, T 300 K
- 1 mol of Ar at p 0.5 atm, T 450 K
- 1 mol of N2 at p 0.5 atm, T 600 K
3
Which system has the largest average
translational kinetic energy?
- 1 mol of He at p 1 atm, T 300 K
- 2 mol of N2 at p 0.5 atm, T 450 K
- 2 mol of He at p 2 atm, T 300 K
- 1 mol of Ar at p 0.5 atm, T 450 K
- 1 mol of N2 at p 0.5 atm, T 600 K
4
Metals such as steel expand when heated. A thin
steel plate has a circular hole in its center.
When the plate is heated, what will happen to the
hole?
(A) gets larger (B) gets smaller (C) stays the
same (D) vanishes
5
Metals such as steel expand when heated. A thin
steel plate has a circular hole in its center.
When the plate is heated, what will happen to the
hole?
(A) gets larger (B) gets smaller (C) stays the
same (D) vanishes
6
What is the ratio Tf /Ti for this process?
- 4
- 2
- 1 (no change)
7
What is the ratio Tf /Ti for this process?
- 4
- 2
- 1 (no change)
8
Two cylinders at the same temperature contain the
same gas. If B has twice the volume and half the
number of moles as A, how does the pressure in B
compare with the pressure in A?
(A) PB 1/2 PA (B) PB 2 PA (C) PB
1/4 PA (D) PB 4 PA (E) PB PA
9
Two cylinders at the same temperature contain the
same gas. If B has twice the volume and half the
number of moles as A, how does the pressure in B
compare with the pressure in A?
(A) PB 1/2 PA (B) PB 2 PA (C) PB
1/4 PA (D) PB 4 PA (E) PB PA
10
A gas cylinder and piston are covered with heavy
insulation. The piston is pushed into the
cylinder, compressing the gas. In this process,
the gas temperature
- doesnt change.
- decreases.
- increases.
- theres not sufficient information to tell.
11
A gas cylinder and piston are covered with heavy
insulation. The piston is pushed into the
cylinder, compressing the gas. In this process,
the gas temperature
- doesnt change.
- decreases.
- increases.
- theres not sufficient information to tell.
12
During an isothermal process, 5.0 J of heat is
removed from an ideal gas. What is the change in
internal energy?
A) zero B) 2.5 J C) 5.0 J D) 10 J
13
During an isothermal process, 5.0 J of heat is
removed from an ideal gas. What is the change in
internal energy?
A) zero B) 2.5 J C) 5.0 J D) 10 J
The Laws of Thermodynamics
14
- If the gas in a container absorbs 300 J of heat,
has 100 J of work done on it, and then does 200 J
of work on its surroundings, what is the increase
in the internal energy of the gas? - (A) 600 J
- (B) 400 J
- (C) 0 J
- (D) 500 J
- (E) 200 J
15
- If the gas in a container absorbs 300 J of heat,
has 100 J of work done on it, and then does 200 J
of work on its surroundings, what is the increase
in the internal energy of the gas? - (A) 600 J
- (B) 400 J
- (C) 0 J
- (D) 500 J
- (E) 200 J
16
- If three identical samples of an ideal gas are
taken from initial state I to final state F along
the paths IAF, IF, and IBF as shown in the
pV-diagram above. Which of the following
statements must be true?(A) Point F is at a
higher temperature than point B.(B) No work is
done by the gas along path IF.(C) The change in
temperature of the gas is the same for all three
paths.(D) The work done by the gas is the same
for all three paths.(E) The expansion along path
IF is isothermal.
17
- If three identical samples of an ideal gas are
taken from initial state I to final state F along
the paths IAF, IF, and IBF as shown in the
pV-diagram above. Which of the following
statements must be true?(A) Point F is at a
higher temperature than point B.(B) No work is
done by the gas along path IF.(C) The change in
temperature of the gas is the same for all three
paths.(D) The work done by the gas is the same
for all three paths.(E) The expansion along path
IF is isothermal.
18
For the two processes shown, which of the
following is true
- QA lt QB.
- QA QB.
- QA gt QB.
19
For the two processes shown, which of the
following is true
- QA lt QB.
- QA QB.
- QA gt QB.
20
- The maximum efficiency of a heat engine that
operates between temperatures of 1000 K in the
firing chamber and 600 K in the exhaust chamber
is most nearly - (A) 33
- (B) 40
- (C) 60
- (D) 67
- (E) 100
21
- The maximum efficiency of a heat engine that
operates between temperatures of 1000 K in the
firing chamber and 600 K in the exhaust chamber
is most nearly - (A) 33
- (B) 40
- (C) 60
- (D) 67
- (E) 100
22
Two objects are made of the same material, but
have different masses and temperatures. If the
objects are brought into thermal contact, which
one will have the greater temperature change?
- (A) the one with the higher initial temperature
- (B) the one with the lower initial temperature
- (C) the one with the greater mass
- (D) the one with the smaller mass
- (E) the one with the higher specific heat
23
Two objects are made of the same material, but
have different masses and temperatures. If the
objects are brought into thermal contact, which
one will have the greater temperature change?
- (A) the one with the higher initial temperature
- (B) the one with the lower initial temperature
- (C) the one with the greater mass
- (D) the one with the smaller mass
- (E) the one with the higher specific heat
24
If you add some heat to a substance, is it
possible for the temperature of the substance to
remain unchanged?
- (A) yes
- (B) no
25
If you add some heat to a substance, is it
possible for the temperature of the substance to
remain unchanged?
- (A) yes
- (B) no
26
You may notice that if a mercury-in-glass
thermometer is inserted into a hot liquid, the
mercury column first drops, and then later starts
to rise (as you expect). How do you explain this
drop?
- (A) the mercury contracts before the glass
contracts - (B) the glass contracts before the mercury
contracts - (C) the mercury contracts before the glass
expands - (D) the glass expands before the mercury expands
- (E) the mercury expands before the glass
contracts
27
You may notice that if a mercury-in-glass
thermometer is inserted into a hot liquid, the
mercury column first drops, and then later starts
to rise (as you expect). How do you explain this
drop?
- (A) the mercury contracts before the glass
contracts - (B) the glass contracts before the mercury
contracts - (C) the mercury contracts before the glass
expands - (D) the glass expands before the mercury expands
- (E) the mercury expands before the glass
contracts
28
A steel ring stands on edge with a rod of some
material inside. As this system is heated, for
which of the following rod materials will the rod
eventually touch the top of the ring?
(A) aluminum (B) steel (C) glass
29
A steel ring stands on edge with a rod of some
material inside. As this system is heated, for
which of the following rod materials will the rod
eventually touch the top of the ring?
(A) aluminum (B) steel (C) glass
30
A grandfather clock uses a brass pendulum to keep
perfect time at room temperature. If the air
conditioning breaks down on a very hot summer
day, how will the grandfather clock be affected?
- (A) clock will run slower than usual
- (B) clock will still keep perfect time
- (C) clock will run faster than usual
31
A grandfather clock uses a brass pendulum to keep
perfect time at room temperature. If the air
conditioning breaks down on a very hot summer
day, how will the grandfather clock be affected?
- (A) clock will run slower than usual
- (B) clock will still keep perfect time
- (C) clock will run faster than usual
32
Two cylinders at the same temperature contain the
same gas. If B has twice the volume and half the
number of moles as A, how does the pressure in B
compare with the pressure in A?
(A) PB 1/2 PA (B) PB 2 PA (C) PB
1/4 PA (D) PB 4 PA (D) PB PA
33
Two cylinders at the same temperature contain the
same gas. If B has twice the volume and half the
number of moles as A, how does the pressure in B
compare with the pressure in A?
(A) PB 1/2 PA (B) PB 2 PA (C) PB
1/4 PA (D) PB 4 PA (D) PB PA
34
In the closed thermodynamic cycle shown in the
P-V diagram, the work done on the gas is
1) positive 2) zero 3) negative
35
In the closed thermodynamic cycle shown in the
P-V diagram, the work done on the gas is
1) positive 2) zero 3) negative
36
The process shown on the T-V graph is an
(A) adiabatic compression. (B) isothermal
compression. (C) isochoric compression. (D)
isobaric compression.
37
The process shown on the T-V graph is an
(A) adiabatic compression. (B) isothermal
compression. (C) isochoric compression. (D)
isobaric compression.
38
An ideal gas is compressed to one-half its
original volume during an isothermal process.
The final pressure of the gas
(A) increases to twice its original value. (B)
increases to less than twice its original value.
(C) increases to more than twice its original
value. (D) does not change.
39
An ideal gas is compressed to one-half its
original volume during an isothermal process.
The final pressure of the gas
(A) increases to twice its original value. (B)
increases to less than twice its original value.
(C) increases to more than twice its original
value. (D) does not change.
40
Is it possible to transfer heat from a hot
reservoir to a cold reservoir?
(A) No. (B) Yes this will happen naturally.
(C) Yes, but work will have to be done. (D)
Theoretically yes, but it hasn't been
accomplished yet.
41
Is it possible to transfer heat from a hot
reservoir to a cold reservoir?
(A) No. (B) Yes this will happen naturally.
(C) Yes, but work will have to be done. (D)
Theoretically yes, but it hasn't been
accomplished yet.
42
Is it possible to transfer heat from a cold
reservoir to a hot reservoir?
(A) No. (B) Yes this will happen naturally.
(C) Yes, but work will have to be done. (D)
Theoretically yes, but it hasn't been
accomplished yet.
43
Is it possible to transfer heat from a cold
reservoir to a hot reservoir?
(A) No. (B) Yes this will happen naturally.
(C) Yes, but work will have to be done. (D)
Theoretically yes, but it hasn't been
accomplished yet.
44
A gas is taken through the cycle illustrated
here. During one cycle, how much work is done on
the gas?
(A) -PV (B) -2PV (C) -3PV (D) -4PV
45
A gas is taken through the cycle illustrated
here. During one cycle, how much work is done on
the gas?
(A) -PV (B) -2PV (C) -3PV (D) -4PV