Subatomic Particles PowerPoint PPT Presentation
1 / 29
Title: Subatomic Particles
1
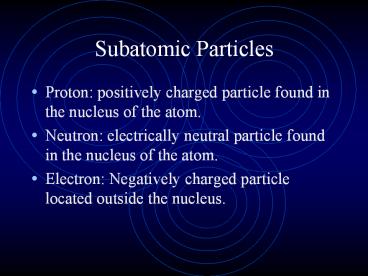
Subatomic Particles
- Proton positively charged particle found in the
nucleus of the atom. - Neutron electrically neutral particle found in
the nucleus of the atom. - Electron Negatively charged particle located
outside the nucleus.
2
Subatomic Particles
Atomic Radius
Nucleus
Electron Cloud
3
Atom
- The smallest particle of an element that retains
the chemical properties of that element.
4
Subatomic Particles
5
Subatomic Particles
ATOM
NUCLEUS
ELECTRONS
PROTONS
NEUTRONS
NEGATIVE CHARGE
POSITIVE CHARGE
NEUTRAL CHARGE
6
Subatomic Particles
- Quarks
- component of protons neutrons
- 6 types
- 3 quarks 1 proton or 1 neutron
7
A. Mass Number
- mass protons neutrons
- always a whole number
- NOT on the Periodic Table!
8
ISOTOPIC NOTATIONisotopes are atoms with the
same number of protons but different number of
neutrons
- A
- Z X
- A mass number
- (the total number of protons
neutrons) - Z atomic number
- (the total number of protons)
- X element symbol
9
B. Isotopes
- Atoms of the same element with different mass
numbers.
- Nuclear symbol
- Hyphen notation carbon-12
10
B. Isotopes
- Chlorine-37
- atomic
- mass
- of protons
- of electrons
- of neutrons
- 17
- 37
- 17
- 17
- 20
11
PRACTICE PROBLEMS
- 15N
- protons ____ neutrons ____
electrons ___ - 35P
- p ____ n ____
e- ___ - 62Cu2
- p ____ n ____
e- ___ - 76Se3-
- p ____ n ____
e- ___
7
8
7
15
20
15
27
29
33
42
34
37
12
Writing ISOTOPIC NOTATION
- Write the symbol for the atom with an atomic
number of 21 and a mass number of 48. - Give the complete chemical notation for the
nuclide with 23 protons, 26 neutrons and 20
electrons. - Write the isotopic notation for
- Z 46 A 110
- An atom containing 24 protons, 28 neutrons, and
21 electrons - Titanium-50
48 Sc
49V3
110Pd
52Cr3
50Ti
13
PRACTICE PROBLEMS
- 196 Pt4
- p _____ n _____ e- _____
- mass number ________ atomic
number _______ - atomic mass ________ name of
element _______ - 2. Indicate the appropriate atomic mass of an
element with 30 protons, 30 neutrons, and 28
electrons.
74
118
78
78
196
195.1 amu
platinum
65.39 amu
14
How Do You Calculate a Weighted Average?
- You have a box of 100 marbles.
- 25 of the marbles have a mass of 2.0g each.
- 75 of the marbles have a mass of 3.0g each.
15
Weighted Average Calculation
- Calculate the total mass of the mixture divide
by 100. 25 x 2.0g 50g 75 x 3.0g
225g - Total mass 275g
- 275g 100 2.75g (average mass of marbles)
16
C. Relative Atomic Mass
- 12C atom 1.992 10-23 g
- atomic mass unit (amu)
- 1 amu 1/12 the mass of a 12C atom
- 1 p 1.007276 amu1 n 1.008665 amu1 e-
0.0005486 amu
17
D. Average Atomic Mass
- weighted average of all isotopes
- on the Periodic Table
- round to 2 decimal places
18
D. Average Atomic Mass
- EX Calculate the avg. atomic mass of oxygen if
its abundance in nature is 99.76 16O, 0.04 17O,
and 0.20 18O.
16.00 amu
19
D. Average Atomic Mass
- EX Find chlorines average atomic mass if
approximately 8 of every 10 atoms are chlorine-35
and 2 are chlorine-37.
35.40 amu
20
Atomic Mass
- The atomic mass of an element represents the
average mass of all the isotopes found in nature.
No element exists with only one possible
isotope. Hydrogen has the smallest number of
isotopes 1H protium, 2H deuterium, 3H tritium.
Its atomic mass is 1.0079 amu (atomic mass
units). The atomic mass is calculated by adding
the of 1H mass found in nature to the of 2H
mass found in nature plus the of 3H mass. - 1H 2H 3H average mass (atomic mass)
- Generally the formula used is
- X Y Z atomic mass.
- An instrument called the mass spectrometer is
generally used to determine the percentages and
individual masses of each isotope.
21
Atomic Mass
- Silver is found to have two stable isotopes, one
has an atomic mass of 106.904 amu and the other
weighs 108.905 amu. The first isotope represents
51.82 of the mass of the element and the second
represents 48.18 . What is the atomic mass of
the element silver? - The equation to use is X Y average
- And remember to turn your percents into fractions
before multiplying. - (0.5182) 106.904 amu (0.4818) 108.905 amu ?
- 55.398 amu 52.470 amu ?
- 107.868 amu !!
- Now look at the periodic table to verify the
answer.
22
PRACTICE PROBLEMS 8
- 1. A sample of neon contains three isotopes,
neon-20 (with an isotopic mass of 19.9924 amu),
neon-21 (20.9939 amu) and neon-22 (21.9914 amu).
The natural abundances of these isotopes are
90.92, 0.257 , and 8.82 . Calculate the
atomic weight of neon. - 2. There are only two naturally occuring
isotopes of copper, 63Cu and 65Cu. Copper has an
atomic mass of 63.55 amu. What is the natural
abundance of each isotope? - 3. There are only two naturally occuring
isotopes of gallium, 69Ga and 71Ga. What is the
natural abundance of each isotope?
20.17 amu
65Cu 27.5 63Cu 72.5
69Ga 64 and 71Ga 36
23
GROUP STUDY PROBLEM 8
- _______1. The element with atomic number 53
contains - a) 53 neutrons b) 53 protons C) 26
neutrons 27 protons d) 26 protons 27
neutrons - _______2. The mass of one atom of an isotope is
9.746 x 10-23 g. One atomic mass unit has the
mass of 1.6606 x 10-24 g. The atomic mass of
this isotope is - a) 5.870 amu b) 16.18 amu c) 58.69 amu d)
1.627 amu - 108
- _______3. The number of neutrons in an atom of
47 Ag is - a) 47 b) 108 c) 155 d) 61
- 27
- _______4. The number of electrons in an ion
of 13 Al3 is - a) 13 b) 10 c) 27 d) 14
- _______5. What is the relative atomic mass of
boron if two stable isotopes of boron have the
following mass and abundance - 10.0129 amu (19.91) 11.0129 (80.09)
- a) 10.81 amu b) 10.21 amu c) 10.62 amu
d) 10.51 amu
24
Calculate the Average Atomic Mass of an Element
(amu)
25
Calculate the Average Atomic Mass of an Element
(amu)
- Copper-63 69.17 x 62.93 amu 4353 amu
- Copper-65 30.83 x 64.93 amu 2002 amu
- Total Mass 6355 amu
- 6355 amu 100 63.55 amu
- What is Coppers Atomic Mass?
26
Calculate the Average Atomic Mass of Oxygen
27
Answer
- 16.00 amu
28
Learning Check
- Naturally occurring carbon consists of three
isotopes, 12C, 13C, and 14C. State the number of
protons, neutrons, and electrons in each of these
carbon atoms. - 12C 13C 14C
- 6 6
6 - p _______ _______
_______ - n _______ _______
_______ - e _______ _______
_______
29
Solution
- 12C 13C 14C
- 6 6
6 - p 6 6 6
- n 6 7 8
- e 6 6 6