TwoComponent Systems PowerPoint PPT Presentation
1 / 18
Title: TwoComponent Systems
1
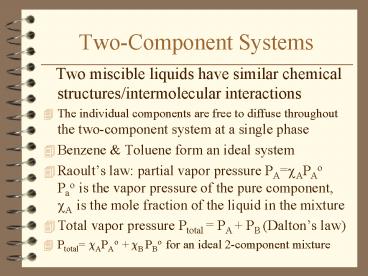
Two-Component Systems
- Two miscible liquids have similar chemical
structures/intermolecular interactions - The individual components are free to diffuse
throughout the two-component system at a single
phase - Benzene Toluene form an ideal system
- Raoults law partial vapor pressure PA?APAo
Pao is the vapor pressure of the pure component,
?A is the mole fraction of the liquid in the
mixture - Total vapor pressure Ptotal PA PB (Daltons
law) - Ptotal ?APAo ?B PBo for an ideal 2-component
mixture
2
Raoults Law for Ideal Two-component System
- Upon mixing, the intermolecular forces are just
the same as those existing in separate components
of the ideal mixture - The energy consumed in overcoming the forces
holding the molecules together in the separate
pure liquids should be provided by the same
forces reforming between the very similar
molecules in the ideal mixture - There is no volume change and no enthalpy change
when an ideal mixture is formed
3
Graphical representation of Raoults Law
Vapor pressure - composition curve for an ideal
mixture
4
Vapor pressure - liquid/vapor composition phase
diagramof an ideal mixture at constant
temperature
- Most ideal solutions contain one component which
is more volatile (higher v.p. when pure) than the
other - The vapor forming above the mixture is richer in
the more volatile component - a represents the liquid composition of a mixture
whose vapor pressure is 1 atm. b represents the
composition of the vapor in equilibrium with the
liquid - b has a larger XB value than a because the
vapor is richer in the more volatile component B.
5
Vapor pressure - liquid/vapor composition phase
diagram of an ideal mixture at constant
temperature
6
From vapor pressure/composition diagram to
boiling point/composition diagram for an ideal
solution
- The normal b.p. of a liquid mixture is the
temperature at which its total vapor pressure is
1 atm. - Vapor pressure (and hence boiling point) of two-
component systems depends on its composition - When XB0.8, the vapor pressure of the mixture is
1 atm. at 70oC so the b.p. for the mixture is
70oC - When XB0.3, vapor pressure of the mixture
reaches 1 atm. at 85oC so the b.p. for the
mixture is 85oC With decreased amount of volatile
component B in a mixture, the b.p. increases
accordingly
7
Variation of vapor pressure/boiling point with
composition Fig.22.3
- At a certain liquid composition, the total vapor
pressure of the ideal solution increases with
temperature. When XB0.3, vapor pressure is 1
atm. at 85oC (b.p.) but reaches 1.2 atm. at 90oC - To find the b.p. of a mixture of given
composition, just read off the temperature at
which the total vapor pressure reaches 1
atmosphere (100 kPa) - At all temperatures above the 1 atm. line the
stable state of the system is as a vapor
8
A boiling point - liquid/vapor composition phase
diagram (at constant pressure) of an ideal
solution
Fig 22.4
9
Applications in Fractional Distillation
- The components of an ideal mixture can be
separated by fractional distillation which makes
use of vapor pressure (b.p.) differences of the
components for their separation from a miscible
liquid mixture - During distillation, the vapor is richer in the
more volatile component as it evaporates
preferentially. - With more volatile component in it, the first
distillate condensed boils at a lower temperature
than the original ideal mixture
10
Fractional distillation
11
The Principle of Fractional Distillation
- Fractional distillation is equivalent to a series
of consecutive simple distillations, where the
condensed vapor from a previous distillation is
used as the liquid for the next distillation. The
lower-boiling condensate from the previous
distillation can be distilled again higher up the
fractionating column at a lower boiling
temperature. The process can be repeated many
times until the final distillate contains mainly
the more volatile component and a negligible
amount of the less volatile component.
12
Deviations from Raoults Law
- The total vapor pressure of a non-ideal liquid
mixture at a constant temperature does not vary
linearly with composition - positive deviation the liquid mixture has vapor
pressure greater than expected from ideal system
the greater tendency to escape arises from
energetically unfavorable interactions upon
mixing (that is, the force of attraction between
A-B molecules is less than that between A-A and
B-B molecules - Ethanol-water, ethanol-chloroform,
ethanol-toluene, ethanol-hexane are systems with
positive deviations
13
Positive deviations from ideality
14
Negative deviation from Raoults Law
- Due to favorable interactions between the
components on mixing, non-ideal solutions show
negative deviation - Mixtures with very large negative deviations have
a minimum in the vapor pressure curve and the
b.p. curve passes through a maximum which is
higher than that of either pure component - At constant pressure, nitric(V) acid water form
a maximum boiling azeotrope the maximum occurs
at 68 nitric acid by mass boiling unchanged at
121oC
15
Fractional distillation of non-ideal solutions
- It is convenient to use boiling point-composition
diagrams at a fixed pressure instead of
vapor-pressure-composition diagrams in
considering fractional distillation of non-ideal
mixtures - For vapor pressure-composition diagrams with no
minimum or maximum, the deviation is relatively
small - Methanol water have very similar structures and
hence identical interactions their vapor
pressure-composition diagram shows no maximum or
minimum - By repeating the boiling-condensing-boiling
process in one operation using a fractionating
column, pure methanol can be obtained from a 10
methanol (90 water) mixture
16
Fractional distillation of non-ideal mixtures
whose boiling-point-composition diagram shows a
maximum
- At the maximum point M the liquid and vapor have
the same composition.That is, if a mixture having
composition M is distilled, the vapor formed at
T1 has exactly the same composition as the liquid
and no separation of the components is achieved. - If a mixture of composition Z is heated, the
vapor initially formed at T2 has a composition Y
which is richer in the more volatile component A,
so that the residue in the flask becomes richer
in the less volatile component B. If this vapor
is then condensed redistilled, the vapor formed
will have composition X, still richer in volatile
A - By fractional distillation, pure A is obtained in
the distillate, leaving the maximum, constant
boiling azeotrope in the flask
17
(No Transcript)
18
(No Transcript)