What is Biochemistry PowerPoint PPT Presentation
1 / 45
Title: What is Biochemistry
1
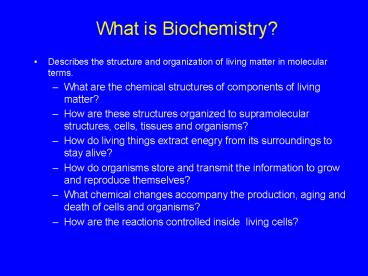
What is Biochemistry?
- Describes the structure and organization of
living matter in molecular terms. - What are the chemical structures of components of
living matter? - How are these structures organized to
supramolecular structures, cells, tissues and
organisms? - How do living things extract enegry from its
surroundings to stay alive? - How do organisms store and transmit the
information to grow and reproduce themselves? - What chemical changes accompany the production,
aging and death of cells and organisms? - How are the reactions controlled inside living
cells?
2
Areas of Biochemistry
- Structural chemistry components of living matter
and relation to biological function to chemical
structure - Metabolism Totality of chemical reactions
occurring in living matter - Information storage and transmission Chemistry
of processes and substances, also includes
molecular genetics (seeks to understand molecular
grounds of heredity and expression of genetic
information)
3
(No Transcript)
4
A Chemical Science Biochemistry
5
Biological Molecules
Immunoglobulin, 150,000 Da
6
A Biological Science Biochemistry
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
(No Transcript)
11
(No Transcript)
12
Microscopy
13
(No Transcript)
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
(No Transcript)
18
The matrix of life Weak interactions in an
Aqueous Environment
19
(No Transcript)
20
(No Transcript)
21
Permenant and induced dipole interactions
Polar or permanent dipole, said to have permanent
dipole moment, ?
22
(No Transcript)
23
(No Transcript)
24
Hydrogen bond
25
(No Transcript)
26
Hydrogen Bonding in Water
27
(No Transcript)
28
(No Transcript)
29
(No Transcript)
30
Amphipathic molecules
31
Interaction of amphipatic molecules in water
32
pH Scale
33
Titration Curves of Weak Acids
34
Molecules with Multiple Ionizing Groups
35
Relative Concentrations of the three forms of
glycine as a function of pH
36
Electrostatic Interactions Between Microions
37
Dependence of Protein Solubility on pH
38
The Influence of Small IonsIonic Strength
39
Chapter 3The Energetics of Life
40
(No Transcript)
41
Equilibration Across a Membrane
42
(No Transcript)
43
(No Transcript)
44
(No Transcript)
45
(No Transcript)