Chapter 5 Ionic Bonds - PowerPoint PPT Presentation
1 / 19
Title:
Chapter 5 Ionic Bonds
Description:
1. Chapter 5 Ionic Bonds. Bonds = attraction of two atoms for the same ... Oxidation = lose electrons, get larger ... A hydrated barium hydroxide salt has a ... – PowerPoint PPT presentation
Number of Views:184
Avg rating:3.0/5.0
Title: Chapter 5 Ionic Bonds
1
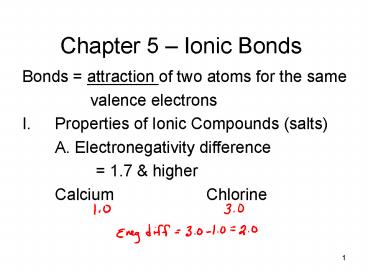
Chapter 5 Ionic Bonds
- Bonds attraction of two atoms for the same
- valence electrons
- Properties of Ionic Compounds (salts)
- A. Electronegativity difference
- 1.7 higher
- Calcium Chlorine
2
- B. formed by transfer of electrons
- -metals lose electrons
- -nonmetals gain electrons
- Oxidation lose electrons, get larger charge
- Ca ? Ca 2 2e-
- Reduction gain electrons, get smaller charge
- Cl 1e-? Cl1-
3
- C. Crystal lattice structure
- not separate molecules
- shape of crystals depends on
- size charges of ions
- D. Hard due to strong bonds
- E. Brittle ionic not lined up
- due to different sizes
4
- F. Very High Density, M.P. B.P.
- (more than metals)
- ions are packed close together
- have charges holding them together
- G. Good conductors if dissolved or melted
- -charges are on the atoms,
- so atoms must move to get charges to
move - H. Soluble in water
- water has and on its molecules
- that attracts and pulls on opposite
charges - in crystal lattice
5
- II. Writing Formulas Naming Ionic Cmpds
- A. Oxidation Numbers
- Group 1 1 Hydrogen 1
- Group 2 2
- Group 13 3
- Group 14 4 or 4- Negative ions
- Group 15 3- -ide ending
- Group 16 2-
- Group 17 1- Hydride 1-
- Group 18 0
- Silver 1 (Group 11)
- Zinc 2 (Group 12)
6
- B. Writing Formulas
- oxidation numbers must add to zero
- so electrons lost electrons gained
- Shortcut Criss-cross Reduce
- empirical formula simplest ratio
- subscript lowered , gives ratio
- Examples
- Silver sulfide
- Magnesium oxide
- Calcium phosphide
7
- C. Transition Metals ( others)
- d-electrons can give more than one
- oxidation number
- use Roman numeral to show charge
- Copper (II) Cu2
- Copper (I) Cu1
- Iron (II) Fe2
- Iron (III) Fe3
- Lead (II) Pb2
- Lead (IV) Pb4
- Example
- Copper (II) oxide
8
- D. Polyatomic Ions
- groups of atoms that act like one ion
- Ammonium NH41
- Hydroxide OH1-
- Acetate C2H3O21-
- Nitrate NO31-
- Chlorate ClO31-
- Carbonate CO32-
- Sulfate SO42-
- Phosphate PO43-
- Use parentheses if extra subscript is needed for
polyatomic ions - Example
- Ammonium sulfate
9
- Naming Ionic Compouds
- 1. ion first (metal or ammonium)
- use Roman numeral for Cu, Fe, Pb
- 2. ion last
- nonmetal elements -ide ending
- polyatomic ions no name change
- Examples
- CuSO4
- Fe2O3
- PbO2
10
- III. Measuring Ionic Compounds
- A. Molar Mass
- total of all atomic masses in a
- compound from periodic table
- grams in 1 mole of compound
- 6.02 x 1023 molecules
11
- Examples
- 1. Find the molar mass of calcium nitrate
- 2. How many moles are in 3.58 grams of
- sodium sulfide?
12
- 3. How many molecules are in 0.24 moles of
- potassium phosphate?
- 4. How many grams are in 1.91 x 1022
- molecules of aluminum sulfate?
13
- B. Percent Composition (by mass)
- part (element) x 100
- whole (compound)
- Remember s should add up to 100
14
- Examples
- 1. Find the composition of
- copper (I) sulfate
- 2. Find the percent of sulfur in
- ammonium sulfide.
15
- IV. Hydrated Salts
- ionic salts with water molecules trapped
- within their crystals
- Anhydrous salts have water removed from them
(usually by heating)
16
- Prefixes in name tell of water molecules
- 1 mono 6 hexa
- 2 di 7 hepta
- 3 tri 8 octa
- 4 tetra 9 nona
- 5 penta 10 deca
- Example
- CaCl2 2H2O
17
- To Calculate Formulas
- 1. Find g water g anhydrous salt
- 2. Convert to moles water
- moles anhydrous salt
- 3. Get whole Mole Ratios
- -divide both by smallest moles
18
- Example
- A hydrated barium hydroxide salt has a mass of
3.15 grams. The anhydrous salt has a mass of 1.71
grams. - 1. Find the formula of the hydrated salt give
its name.
19
- 2. Find the percent of water in the hydrated
salt. - 3. Find the molar mass of the hydrated salt.