CarbonCarbon bonds: cis and trans isomers PowerPoint PPT Presentation
1 / 19
Title: CarbonCarbon bonds: cis and trans isomers
1
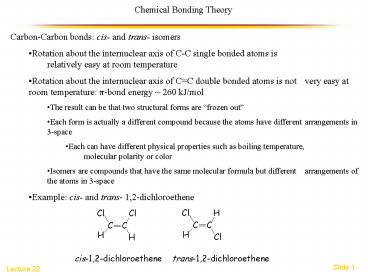
Chemical Bonding Theory
- Carbon-Carbon bonds cis- and trans- isomers
- Rotation about the internuclear axis of C-C
single bonded atoms is relatively easy at room
temperature - Rotation about the internuclear axis of CC
double bonded atoms is not very easy at room
temperature p-bond energy 260 kJ/mol - The result can be that two structural forms are
frozen out - Each form is actually a different compound
because the atoms have different arrangements in
3-space - Each can have different physical properties such
as boiling temperature, molecular polarity or
color - Isomers are compounds that have the same
molecular formula but different arrangements of
the atoms in 3-space - Example cis- and trans- 1,2-dichloroethene
2
(No Transcript)
3
Chemical Bonding Theory
- Delocalized p bonds When two or more resonance
structures can be written involving p bonds, the
p bonds can be represented as being spread out
over the molecule where the p bonds occur. - The electrons and the bonds containing them are
delocalized. - Example Benzene
The s framework of C-C and C-H bonds is based
on sp2 hybridized C atoms. a. After accounting
for s bonding, an unhybridized p orbital
remains on each C atom, with one electron per p
orbital b. The p orbitals overlap to form p
bonds that form a continuous p electron cloud
above and below the plane of the ring
Delocalized p bonds
4
Chemical Bonding Theory
- Summary of Valence Bond Results
- Bonded atoms share one or more pairs of electrons
- At least one s bond exists between each pair of
bonded electrons. - s bonds are cylindrically symmetric along the
internuclear axis and electrons are concentrated
- localized - between the bonded atoms. - An appropriate set of hybrid atomic orbitals is
formed to form s bonds. - The set of hybrid orbitals depends on the number
of s bonds to be formed, the number of
nonbonded electron pairs and the geometry of the
molecule. - AX2 type sp hybridization linear molecular
geometry - AX3 or AX2E type sp2 hybridization trigonal
planar or bent geometry - AX4, AX3E or AX2E2 type sp3 hybridization
tetrahedral, trigonal pyramidal, bent molecular
geometry. - AX5, AX4E, AX3E2, AX2E3 type sp3d hybridization
trigonal bipyramid, see saw, T shape or linear
molecular geometry. - AX6, AX5E, AX4E2 type sp3d2 hybridization
octahedral, square pyramid, square planar
molecular geometry.
5
Chemical Bonding Theory
- Summary of Valence Bond Results
- Atoms sharing more than one pair of electrons
form p bonds by sideways overlap of p atomic
orbitals. - p bonds have a nodal plane containing the
internuclear axis - In p bonds, electron density is concentrated
above and below the nodal plane. - Molecules with two or more resonance structure
can have p bonds delocalized over more than two
atoms.
6
Chemical Bonding Theory
- Molecular Orbital Theory (MO theory)
- This method deals with interactions of shared
electrons in a different way. - Molecular orbitals are formed in such a way that
they cover the entire molecule. - The method used is to form Linear Combinations of
Atomic Orbitals - LCAOs - There are 2 such combinations from any two atomic
orbitals - yMO a1yAO 1 a2yAO 2
- yMO a1yAO 1 - a2yAO 2
- The as indicate how much of each AO is involved
in yMO. In our case, the as are 1. - The 1st principle of MO theory is that the total
number of molecular orbitals is always equal to
the total number of atomic orbitals contributed
by the atoms combined - Other more complicated LCAOs can be constructed
as more AOs are combined
7
Chemical Bonding Theory
- MO Theory
- Two results obtain from MO theory
- The shapes of the molecular orbitals can be
determined. - The energies of the molecular orbitals can be
determined. - Example H2 molecule
yMO y1s H1 y1s H2 s1s yMO y1s H1 - y1s
H2 s1s
2nd principle of MO theory the bonding MO is
lower in energy than the antibonding MO
s1s2s1s 0
s1s orbital is a bonding orbital - there is
electron density between atoms s1s orbital is an
antibonding MO - there is a nodal plane
perpendicular to the internuclear axis between
the nuclei
8
(No Transcript)
9
- Chemical Bonding Theory
- MO Theory
- Energy Level Diagram for H2
- Bond order 1( bonding electrons - antibonding
electrons) - Energy Level Diagram for He2 Energy
Level Diagram for He2 - s1s2 s1s1 B. O. 1
s1s2 s1s2
B. O. 0 - paramagnetic
3rd principle of MO theory electrons of a
molecule are assigned to orbitals of
successively higher energy according to the
Pauli exlusion principle and Hunds rule of
maximum spin multiplicity
10
Chemical Bonding Theory
- MO Theory
- 2nd Period Homonuclear Diatomic Molecules
- Li2 Be2
s1s2 s1s2 s2s2 B. O. 1 s1s2 s1s2
s2s2 s2s2 B. O. 0
diamagnetic
11
- Chemical Bonding Theory
- MO Theory
- 2nd Period Homonuclear Diatomic Molecules
- LCAOs for 2p orbitals
- Head-to-head LCAOs
- Sideways overlap
There is a 2nd set of p orbitals perpendicular
to to those shown.
12
(No Transcript)
13
(No Transcript)
14
- Chemical Bonding Theory
- MO Theory
- 2nd Period Homonuclear Diatomic Molecules
- B2 C2
s1s2 s1s2 s2s2s2s2p2p2 B. O. 1 s1s2
s1s2s2s2s2s2p2p4 B. O. 0
paramagnetic
15
- Chemical Bonding Theory
- MO Theory
- 2nd Period Homonuclear Diatomic Molecules
- N2 O2
s1s2 s1s2 s2s2s2s2p2p4s2p2 B. O. 3 s1s2
s1s2 s2s2s2s2s2p2p2p4p2p2 B. O. 2
diamagnetic paramagnetic
16
- Chemical Bonding Theory
- MO Theory
- 2nd Period Homonuclear Diatomic Molecules
- F2 Ne2
s1s2 s1s2 s2s2s2s2s2p2p2p4p2p4 B. O. 1 s1s2
s1s2 s2s2s2s2s2p2p2p4p2p4s2p2 B. O.0
diamagnetic
17
Chemical Bonding Theory
- MO Theory
- Heteronuclear diatomics
- The MO treatment is very similar to that for the
homonuclear diatomics - Use the same energy level diagram for F2, except
that the more electronegative element has AOs
at lower energy than the less electronegative
element - Example CO
- s1s2 s1s2 s2s2s2s2s2p2p2p4
- B. O. 3
- Diamagnetic
18
Chemical Bonding Theory
- MO Theory
- Delocalization of p electrons - the MO analogy to
resonance - In species such as O3, NO2-, CO32-, benzene,
resonance was used in valence bond theory to
explain the equivalence of the multiply bonded
atoms - MO theory makes use of electron delocalization of
the p electrons to explain the same observation
- Example O3
- Assume the central atom is trigonal planar
- The central atom makes 2 s bonds with the
terminal Os - Each atom has 3 unused p orbitals having p
symmetry containing 4 electrons - The 3 p orbitals form 3 MOs a p bonding MO, a p
antibonding MO and a p nonbonding MO - The 4 electrons form p MO electron configuration
p2 pnb2 pp0 - The p bond order is 0.5, giving a total average
O-O bond order of 1.5
19
Chemical Bonding Theory
- MO Theory
- Delocalization of p electrons - the MO analogy to
resonance - Example Benzene
- The six p orbitals on C having p symmetry form
6 p MOs whose energy diagram is shown - The three p bonding MOs extend over the 6
carbons in the ring resulting in electron
delocalization over the ring of carbon