Add this to your notes! - PowerPoint PPT Presentation
1 / 25
Title:
Add this to your notes!
Description:
Color the nitrogen family with color 7 Nitrogen Family Elements in group 15 OXYGEN FAMILY or Chalcogens Group 16 O, S, Se, Te, Po Gas (O) ... – PowerPoint PPT presentation
Number of Views:55
Avg rating:3.0/5.0
Title: Add this to your notes!
1
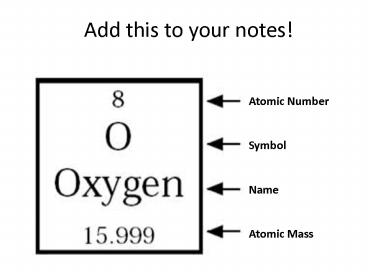
Add this to your notes!
Atomic Number Symbol Name Atomic Mass
2
How do you find the of protons, electrons and
neutrons using the periodic table?
- Atomic Number of Protons
- Atomic Number of Electrons
- of Neutrons Atomic Mass Atomic Number
3
Bohr Diagrams
- A 2-dimensional display of an atom.
- NOT perfect by any means remember atoms are 3D
and electrons do not move in orbits - Each circle represents an energy level.
- The energy of the electrons increases as they get
farther from the nucleus. - Level 1 up to 2 electrons
- Level 2 up to 8 total electrons
- Level 3 up to 18 total electrons
- Level 4 up to 32 total electrons
- Levels 2, 3 and 4 have sublevels the electrons
would not be found in 1 zone but rather multiple
zones.
4
- You need 11 different colors
5
Families on the Periodic Table
- Elements on the periodic table can be grouped
into families bases on their chemical properties. - Each family has a specific name to differentiate
it from the other families in the periodic table. - Elements in each family
- react differently with
- other elements.
- The horizontal rows are
- called periods and are
- labeled from 1 to 7.
- The vertical columns are called groups or
families and are labeled from 1 to 18.
6
Hydrogen
- Hydrogen belongs to a family of its own.
- Hydrogen is a diatomic, reactive gas.
- Diatomic always found as a 2-atom molecule
- Reactive has a tendency to react chemically
- Hydrogen was involved in the explosion of the
Hindenberg. - Hydrogen is promising as an alternative fuel
source for automobiles - color hydrogen color 1
7
ALKALI METALS
- Group 1
- Hydrogen is not a member, it is a non-metal
- Li, Na, K, Rb, Cs, Fr
- All solids
- 1 electron in the outer shell
- All metals
- Always combined with something else in nature
Very reactive, esp. with water - (ex. Salt)
- Soft (can cut with butter knife) and silvery
metals - Conduct electricity
- Color the rest of this group color 2
8
ALKALINE EARTH METALS
- Group 2
- Be, Mg, Ca, Sr, Ba, Ra
- All solids
- 2 electrons in the outer shell
- All metals
- Reactive, but less than Alkali metals
- Facts
- White and malleable
- Malleable able to be hammered or pressed out of
shape without breaking. - Conduct electricity
- Always combine with nonmetals in nature
- Important nutrients for humans (Mg, Ca)
- Color the Alkaline Earth Metals color 3
9
TRANSITION METALS
- Groups 3-12
- Mostly Solids, 1 liquid (Hg)
- 1 or 2 valence electrons
- All metals, tend to be harder
- Less reactive.
- These are what you think of as metals.
- Good conductors of heat and electricity.
- Some are used for jewelry.
- Some are used in construction.
- Can bond with many elements in a variety of
shapes. - Color Transition Metals color 4
10
Transition Metals
11
BORON FAMILY
- Group 13
- B, Al, Ga, In, Tl
- All solids
- 3 electrons in the outer shell
- Most are metals
- Boron is a metalloid
- Metalloid has properties of both metals and
nonmetals - Moderately reactive
- Includes Aluminum, the most abundant metal on
Earth. - Color Boron Family color 5
12
CARBON FAMILY
- Group 14
- C, Si, Ge, Sn (Tin), Pb (Lead)
- All solids
- 4 electrons in the outer shell
- Contains metals, metalloids, and a non-metal
Carbon (C) - Non-reactive.
- Facts
- Contains elements important to life and
computers. - Carbon is the basis for an entire branch of
chemistry. - Silicon and Germanium are important
semiconductors. - Color Carbon Family with color 6
13
NITROGEN FAMILY
- Group 15
- N, P, As, Sb, Bi
- Solids except for N (gas)
- 5 electrons in the outer shell
- Contains 1 metal, 2 metalloids, and 2 non-metals
- Generally non-reactive
- Facts
- Can share electrons to form compounds, called
covalent bonds. - Nitrogen makes up over ¾ of the atmosphere.
- The red stuff on the tip of matches is
phosphorus. - Color the nitrogen family with color 7
14
Nitrogen Family
- Elements in group 15
15
OXYGEN FAMILY or Chalcogens
- Group 16
- O, S, Se, Te, Po
- Gas (O) and Solids
- 6 electrons in the outer shell
- Contains metals, metalloids, and non-metals
- Reactive
- Facts
- Oxygen is necessary for respiration.
- Many things that stink, contain sulfur (rotten
eggs, garlic, skunks,etc.) - Color the Oxygen family with color 8
16
Oxygen Family or Chalcogens
- Many things that stink, contain sulfur (rotten
eggs, garlic, skunks,etc.)
17
Halogens
- Group 17
- F, Cl, Br, I, At
- Solids (I, At), Liquids (Br), Gases (O, Cl)
- 7 electrons in the outer shell
- All are non-metals
- Very reactive, volatile, diatomic, nonmetals
- Facts
- Always found combined with other element in
nature. Often bonded with elements from group 1. - Used as disinfectants and to strengthen teeth.
- Color the Halogen group with color 9
18
Noble Gases
- Group 18
- He, Ne, Ar, Kr, Xe, Rn
- All gases
- Non-metals
- 8 electrons in the outer shell Full EXCEPT
Helium (has 2 electrons in outer shell but is
considered full) - NOT reactive with other elements
- Color the noble gases with color 10
19
Metal/ Nonmetal/Metalloid
- Choose 3 markers of different colors.
- Outline the following elements in color 1
- The elements to the LEFT of the line are
metalloids. Outline the metalloids in 1 dark
color other than black. Include Ge and Sb in the
outline. - The elements to the Right of the zigzag are
nonmetals. Outline those in another dark color. - The metals to the left of the metaloids are all
metals. Outline those in a 3rd dark color. - Make a key on the bottom of your table for
metals, nonmetals and metalloids.
20
Metal/ Nonmetal/Metalloid
- Choose 3 markers of different colors.
- Outline the following elements in color 1
- The elements to the LEFT of the line are
metalloids. Outline the metalloids in 1 dark
color other than black. Include Ge and Sb in the
outline. - The elements to the Right of the zigzag are
nonmetals. Outline those in another dark color. - The metals to the left of the metaloids are all
metals. Outline those in a 3rd dark color. - Make a key on the bottom of your table for
metals, nonmetals and metalloids.
21
Metals, Metalloids, Non-Metals
- METALS
- Luster (Shiny)
- Good conductor of heat
- Good conductor of electricity
- Malleable (can be bent and shaped)
- Ductile (can be drawn into wire)
- Corrosive (tendency to deteriorates under certain
chemical conditions. ie rusting iron, pennies
turning green). - Usually dense
- Usually solid
- Have high melting points
- Generally Reactive
- Left side of periodic table.
22
Metals, Metalloids, Non-Metals
- METALLOIDS
- Dull or Shiny
- Good semiconductors (that means conducts heat or
electricity under certain conditions) - Sometimes malleable
- Sometimes ductile
- Generally solid at room temperature (but found as
all three states of matter.) - Makes the zigzag on the periodic table.
23
Metals, Metalloids, Non-Metals
- NON-METALS
- Dull appearance
- Brittle (breaks or shatter easily)
- Poor conductors of heat.
- Poor conductors of electricity.
- Less dense
- Low melting points
- All three states of matter
- Gain or share electrons when they react with
other element. - Right side of periodic table.
24
Periodic Table Scavenger HuntYou need a piece of
paper for this
- I have 26 protons and am a transition metal.
- Im the only Metalloid in the oxygen family.
- I am the only metal that is a liquid.
- I am in period 4 and have 8 valence electrons.
- I am in group 2 and have 56 protons.
- I am a nonmetal with 4 valence electrons.
- Im a metalloid with 14 neutrons in group 14.
- I am the nonmetal next to the metalloid in group
16. - I am the Noble Gas with the least protons.
- I am the only nonmetal in period 6.
25
Periodic Table Scavenger Hunt
- I have 26 protons and am a transition metal.
-Iron (Fe) - Im the only Metalloid in the oxygen family. -Te
(Tellurium) - I am the only metal that is a liquid. - Hg
(Mercury) - I am in period 4 and have 8 valence electrons. -
Kr Krypton - I am in group 2 and have 56 protons. - Ba Barium
- I am a nonmetal with 4 valence electrons. C
(carbon) - Im a metalloid with 14 neutrons in group 14.
Si (Silicon) - I am the nonmetal next to the metalloid in group
16. I (Iodine) - I am the Noble Gas with the least protons. He
(Helium) - I am the only nonmetal in period 6. Rn (Radon)




![READ⚡[PDF]✔ Notes on a Nervous Planet PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10069994.th0.jpg?_=20240702106)



![get [PDF] Download 2025 Love Notes from Chippy Boxed Calendar: A Year of Heartfelt Messages from PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10081262.th0.jpg?_=20240718025)
![❤[PDF]⚡ I've F*cking Got This Sticky Notes: 101 Affirmations to Swear and Share, a PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10062888.th0.jpg?_=20240624075)




















