Ground state - PowerPoint PPT Presentation
1 / 19
Title:
Ground state
Description:
Ef = Ei hn. The Law of Conservation of Energy requires that: Lab: 4/15. 4/16. Line spectrum ... Periodic trends are explained. by the distribution of ... – PowerPoint PPT presentation
Number of Views:21
Avg rating:3.0/5.0
Title: Ground state
1
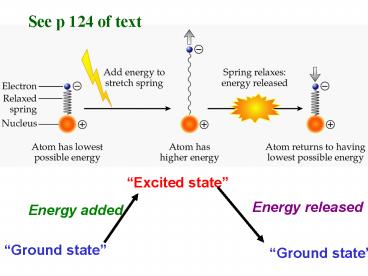
See p 124 of text
Excited state
Energy released
Energy added
Ground state
Ground state
2
See p 129-132 of text
Excited state
Ground state
3
The Law of Conservation of Energy requires that
A quantum jump
For absorbance Ef Ei hn
For emission Ef Ei - hn
4
Lab 4/15 4/16
5
Line spectrum of Neon gas See p 108 of text
6
What YOU should know
- Rutherford model of atom
- Bohr model of atom
- Differences and similarities between Bohr and
Rutherford models - What is a quantum jump?
- What is a line spectrum? What information does
it provide with respect to atomic structure?
7
What YOU should know
- How to calculate wavelength of light that is
emitted (or absorbed) when an electron jumps from
one energy level to another - How to calculate a value for the energy of light
when you are given its wavelength (for lines in a
line spectrum this value corresponds to the
energy difference between two electron orbitals
in an atom)
8
(No Transcript)
9
See p 101 of text
See p 126 of text
Periodic trends are explained by the
distribution of electrons in orbitals!!
10
See p 103 of text
11
See p 126 of text
12
Neutral atoms can gain or lose electrons to form
ions
See p 103 of text
13
See p 104 of text
14
See p 126 of text
15
See p 126 of text
16
Ionization energy
- minimum energy required (in units of kJ/mol) to
remove the highest energy (i.e. the outermost)
electron from a neutral atom to form a cation - Li ? Li e-
17
See p 105 of text
18
See p 106 of text
19
See p 106 of text