What disease is associated with Koplik Spots bluewhite spots on buccal mucous membranes 2448 prior t - PowerPoint PPT Presentation
1 / 103
Title:
What disease is associated with Koplik Spots bluewhite spots on buccal mucous membranes 2448 prior t
Description:
Combination measles, mumps, rubella and varicella vaccine ... Measles and mumps viruses grown in chick embryo fibroblast culture ... – PowerPoint PPT presentation
Number of Views:174
Avg rating:3.0/5.0
Title: What disease is associated with Koplik Spots bluewhite spots on buccal mucous membranes 2448 prior t
1
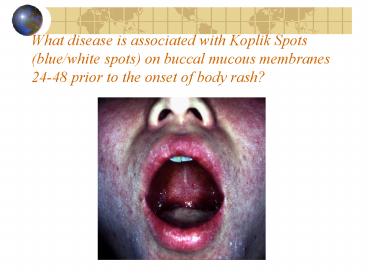
What disease is associated with Koplik Spots
(blue/white spots) on buccal mucous membranes
24-48 prior to the onset of body rash?
2
Rash Associated with this disease?
3
Similar Rash in a Child in Nigeria
4
Measles Epidemiology
- Reservoir Human
- Transmission Respiratory Airborne
- Temporal pattern Peak in late winterspring
- Communicability 4 days before to 4 days
after rash onset
5
MeaslesUnited States, 1950-2005
- Vaccine Licensed
2005 provisional total
6
Available Measles Vaccine
- Measles Vaccine - Attenuvax
- Measles Rubella Vaccine
- M-R-Vax II
- Measles, Mumps Rubella Vaccine M-M-R II
7
Which disease causes this classic symptom?
8
Mumps Epidemiology
- Reservoir Human Asymptomatic
infections may transmit - Transmission Respiratory drop nuclei
- Temporal pattern Peak in late winter and spring
- Communicability Three days before to four
days after onset of active disease
9
Mumps Vaccine
- Composition Live virus (Jeryl Lynn strain)
- Efficacy 95 (Range, 90-97)
- Duration ofImmunity Lifelong
- Schedule gt1 Dose
- Should be administered with measles and rubella
(MMR) or with measles, rubella and varicella
(MMRV) - Mumps vaccine is available for unique situations
(Mumpsvax)
10
Symptoms of which disease?
11
Rubella Epidemiology
- Reservoir Human
- Transmission Respiratory Subclinical cases
may transmit - Temporal pattern Peak in late winter and spring
- Communicability 7 days before to 5-7 days
- after rash onset Infants with CRS may
shed virus for a year or more
12
Epidemic Rubella United States, 1964-1965
- 12.5 million rubella cases
- 2,000 encephalitis cases
- 11,250 abortions (surgical/spontaneous)
- 2,100 neonatal deaths
- 20,000 Congenital Rubella Syndrom (CRS) cases
- deaf - 11,600
- blind - 3,580
- mentally retarded - 1,800
13
Cataracts Caused by CRS
14
Rubella and CRS in the U. S.
- Most reported rubella in the U.S. since the
mid-1990s has occurred among foreign-born
Hispanic adult - Majority of CRS since 1997 occurred in children
of unvaccinated women born to Hispanic women,
most born in Latin America
15
Rubella Vaccine
- Composition Live virus (RA 27/3 strain)
- Efficacy 95 (Range, 90-97)
- Duration ofImmunity Lifelong
- Schedule gt1 Dose
- Should be administered with measles and mumps as
MMR or with measles, mumps and varicella as MMRV
16
Measles (MMR) Vaccine Indications
- All infants gt12 months of age
- Susceptible adolescents and adults without
documented evidence of immunity
17
MMRV (ProQuad)
- Combination measles, mumps, rubella and varicella
vaccine - Approved children 12 months through 12 years of
age (up to age 13 years) - Titer of varicella vaccine virus in MMRV is more
than 7 times higher than standard varicella
vaccine
18
Measles Mumps Rubella Vaccine
- Live vaccine
- Two doses 0.5ml SQ minimum interval 4 wks.
- 12 months is the recommended and minimum age
2nd dose at 4-6 yrs. - MMR given before 12 months should not be counted
as a valid dose - Revaccinate at gt12 months of age
19
MMR Adverse Reactions
- Fever 5-15
- Rash 5
- Joint symptoms 25
- Thrombocytopenia lt1/30,000 doses
- Parotitis rare
- Deafness rare
- Encephalopathy lt1/1,000,000 doses
20
MMR VaccineContraindications and Precautions
- Severe allergic reaction to vaccine component or
following prior dose - Pregnancy
- Immunosuppression
- Moderate or severe acute illness
- Recent blood product
21
Measles and Mumps Vaccines and Egg Allergy
- Measles and mumps viruses grown in chick embryo
fibroblast culture - Studies have demonstrated safety of MMR in egg
allergic children - Vaccinate without testing
22
Vaccine Storage and HandlingMMR Vaccine
- Must be shipped to maintain a temperature of lt-
4oF (-20oC ) at all times - Must be stored at an average temperature of lt5oF
(-15oC ) at all times - May NOT be stored at refrigerator temperature at
any time - Must be administered within 30 minutes of
reconstitution
23
Adolescent Female with Varicella Lesions in
Various Stages
24
Varicella Lesions on Face Palate
25
Varicella Lesions on Sclera
26
Herpes Zoster (Shingles) Compare to
Chickenpox Lesions
- Chickenpox
- Shingles
27
Herpes Zoster Shingles- in an Elderly Woman
28
Varicella Epidemiology
- Reservoir Human
- Transmission Airborne droplet Direct contact
with lesions - Temporal pattern Peak in winter and early
spring (U.S.) - Communicability 1-2 days prio to 4-5
days after onset of rash May be longer
in immunocompromised
29
Herpes Zoster
- Reactivation of varicella zoster virus
- Associated with
- aging
- immunosuppression
- intrauterine exposure
- varicella at lt18 months of age
30
Varicella-Containing Vaccines
- Varicella vaccine (Varivax)
- Approved for person 12 months and older
- Measles-mumps-rubella-varicella vaccine (ProQuad)
- Approved for children 12 months through 12 years
- Herpes zoster vaccine (Zostavax)
- Approved for persons 60 years and older
31
Varicella Vaccine (Varivax Merck)
- Composition LIVE virus
- Efficacy 95 (Range, 65-100)
- Dosage Children (1-12 yrs) A single 0.5ml dose
- Adults Adolescents gt13 yrs Two doses
second dose 4-8 weeks after 1st dose - Indication Approved for use in patients 12
months of age and older - May be administered simultaneously with
measles, mumps, and rubella (MMR) vaccine
32
MMRV vaccine
- Approved for children 12 months through 12 years
of age (to 13 years) - Do not use for persons 13 years and older
- May be used for both first and second doses of
MMR and varicella vaccines - Minimum interval between doses is 3 months
33
Herpes Zoster vaccine Zostavax - Merck
- Approved in May of 2006
- For use in person 60 years of age and older
- Contains the same varicella virus as the other
vaccines but is 14 times more potent then the
Varivax - Single 0.65 ml dose administered subcutaneously
preferably in the upper arm
34
Vaccine Storage and HandlingVaricella Vaccine
- Store frozen at 5F (-15C ) or lower
- Store diluent at room temperature or refrigerate
- Discard if not used within 30 minutes of
reconstitution
35
Varicella Vaccine Adverse Reactions
- Injection site complaints 19
(children) 24
(adolescents and adults)
- Rash 3-4
- may be maculopapular ratherthan vesicular
- average 5 lesions
- Systemic reactions not common
36
Varicella Vaccine RecommendationsAdolescents and
Adults
- All persons gt13 years of age without evidence of
varicella immunity - Two doses separated by 4-8 weeks
- Do not repeat first dose because of extended
interval between doses
37
Varicella VaccinePostexposure Prophylaxis
- Varicella vaccine is recommended for use in
persons without evidence of varicella immunity
after exposure to varicella - 70-100 effective if given within 72 hours of
exposure - not effective if gt5 days but will produce
immunity if not infected
38
Jaundice in Sclera Related to Hepatitis A
39
Hepatitis A Clinical Features
- Incubation period 28 days (range 15-50 days)
- Illness not specific for hepatitis A
- Likelihood of symptomatic illness directly
related to age - Children generally asymptomatic, adults
symptomatic
40
Hepatitis A Clinical Features
- Sxs Fever, loss of appetite, nausea, stomach
pain, dark urine, and yellowing of the skin and
whites of the eyes. - Sudden onset can last up to 2 months, 15 of
patients have symptoms for up to 12 months. - Adults with symptoms require hospitalization in
11-22 of cases and are absent from work an
average of 27 days. - The likelihood of developing symptoms of
hepatitis A is related to one's age. Children lt
six years of age have no symptoms but 70 of
adults do have symptoms.
41
- Hepatitis AUnited States, 1966-2005
Year
2005 provisional total
42
Hepatitis AUnited States, 1990-2000 Risk Factors
Source NNDSS/VHSP
43
Hepatitis A Epidemiology
- Reservoir Human
- Transmission Fecal-oral
- Temporal pattern None
- Communicability 2 weeks before to 1 week
after onset
44
Hepatitis A Vaccines
- Inactivated whole virus
- HAVRIX (GlaxoSmithKline)
- VAQTA (Merck)
- Pediatric and adult formulations
- Licensed for persons gt12 months of age
45
- Hepatitis A Vaccines
Formulation Pediatric age dose Adult
age dose
HAVRIX 1-18 yrs 0.5 ml gt18 yrs 1.0 ml
VAQTA 1-18 yrs 0.5 ml gt18 yrs 1.0 ml
46
Hepatitis A Vaccines
Adult
- - 1 dose
- - booster dose 6-18 months after first dose
- - 1 dose
- - booster dose 6-18 months after first dose
Children and Adolescent
47
Hepatitis A Vaccine Recommendations
- International travelers
- Men who have sex with men
- Persons who use illegal drugs
- Persons who have clotting-factor disorders
- Persons with occupational risk
- Persons with chronic liver disease
48
Hepatitis A Vaccine Recommendations
- Healthcare workers not routinely recommended
- Child care centers not routinely recommended
- Sewer workers or plumbers not routinely
recommended - Food handlers may be considered based on local
circumstances
49
Hepatitis B Virus Infection
- gt300 million chronically infected worldwide
- Established cause of chronic hepatitis and
cirrhosis - Human carcinogencause of up to 80 of
hepatocellular carcinomas
50
Hepatitis B Clinical Features
- Incubation period 60-150 days (average 90 days)
- Nonspecific prodrome of malaise, fever, headache,
myalgia - Illness not specific for hepatitis B
- At least 50 of infections asymptomatic
51
Hepatitis B Complications
- Fulminant hepatitis
- Hospitalization
- Cirrhosis
- Hepatocellular carcinoma
- Death
52
Hepatitis B Epidemiology
- Reservoir Human
- Transmission Bloodborne
Subclinical cases transmit - Communicability 1-2 months before and after
onset of symptoms
Chronic carriers
53
HBV Disease Burden in the United States
- New infections 78,000/yr
- Current carriers gt1 million
- New carriers gt5,000/yr
- Death 5,000/yr
2001 estimates
54
Strategy to Eliminate Hepatitis B Virus
TransmissionUnited States
- Prevent perinatal HBV transmission
- Routine vaccination of all infants
- Vaccination of children in high-risk groups
- Vaccination of adolescents
- Vaccination of adults in high-risk groups
55
Hepatitis B Vaccine
- Composition Recombinant HBsAg
- Efficacy 95 (Range, 80-100)
- Duration ofImmunity gt15 years
- Schedule 3 Doses Adults IM,
- Children Anteriolateral Thigh
- Booster doses not routinely recommended
56
Hepatitis B Vaccine Formulations
- Recombivax HB (Merck) - 5 mcg/0.5 mL
(pediatric) - 10 mcg/1 mL (adult) - 40 mcg/1 mL
(dialysis) - Engerix-B (GSK) - 10 mcg/0.5 mL (pediatric) -
20 mcg/1 mL (adult) - Dosing See product info - varies
- (0, 1 6 mo. common with Recombivax)
57
- Recommended Dose of Hepatitis B Vaccine
Recombivax HB Dose (mcg) 0.5 mL (5) 0.5 mL
(5) 1.0 mL (10)
Engerix-B Dose (mcg) 0.5 mL (10) 0.5 mL
(10) 1.0 mL (20)
Infants and children lt11 years of
age Adolescents 11-19 years Adults gt20 years
58
Twinrix
- Combination hepatitis A vaccine (pediatric dose)
and hepatitis B (adult dose) - Schedule 0, 1, 6 months
- Approved for persons gt18 years
59
COMVAX
- Hepatitis B-Hib combination
- Use when either antigen is indicated
- Cannot use lt6 weeks of age
- May be used in infants whose mothers are HBsAg
positive or status is unknown
60
Pediarix
- DTaP Hep B IPV combination
- Approved for 3 doses at 2, 4 and 6 months
- Not approved for booster doses
- Licensed for children 6 weeks to 7 years of age
61
Hepatitis B VaccineAdverse Reactions
Infants and Children 3-9 0-20 0.4-6 rare
Adults 13-29 11-17 1 rare
- Pain at injection site
- Mild systemic complaints(fatigue, headache)
- Temperature 99.9F (37.7C)
- Severe systemic reactions
62
Influenza Virus Strains
- Type A - moderate to severe illness - all age
groups - humans and other animals - Type B - milder disease - primarily affects
children - humans only - Type C - rarely reported in humans - no
epidemics
63
Influenza Pathogenesis
- Respiratory transmission of virus
- Replication in respiratory epithelium with
subsequent destruction of cells - Viremia rarely documented
- Viral shedding in respiratory secretions for 5-10
days
64
Influenza Clinical Features
- Incubation period 2 days (range 1-4 days)
- Severity of illness depends on prior experience
with related variants - Abrupt onset of fever, myalgia, sore throat,
nonproductive cough, headache
65
Influenza Complications
- Pneumonia
- secondary bacterial
- primary influenza viral
- Reyes syndrome
- Myocarditis
- Death 0.5-1 per 1,000 cases
66
Influenza Epidemiology
- Reservoir Human, animals (type A only)
- Transmission Respiratory Probably airborne
- Temporal pattern Peak December March in
temperate climate May occur earlier or later - Communicability 1 day before to 5 days after
onset (adults)
67
Impact of Influenza
- 36,000 excess deaths per year
- gt90 of deaths among persons gt65 years of age
- Higher mortality during seasons when influenza
type A (H3N2) viruses predominate
68
Impact of Influenza
- Highest rates of complications and
hospitalization among young children and person
gt65 years - Average of gt200,000 influenza-related excess
hospitalizations - 57 of hospitalizations among persons lt65 years
of age - Greater number of hospitalizations during type A
(H3N2) epidemics
69
Month of Peak Influenza Activity United States,
1976-2005
45
21
14
10
3
3
70
Influenza Vaccines
- Inactivated, viral subunits split-virus (IIV)
- 1. Fluvirin (Chiron)
- 2. Fluzone (Sanofi Pasteur)
- 3. Fluarix (GSK) Prefilled 0.5ml syringes
- intramuscular
- trivalent
- Live attenuated vaccine (LAIV)
- - Brand Name FluMist
- intranasal
- trivalent
71
Comparison of Influenza Vaccines
- Inactivated vaccine (IIV)
- Dose 0.5ml
- Route IM (Adults Deltoid,
- Children Anteriolateral aspect of thigh)
- Labeled Age Range 6 mo or older
(Fluvirin/Chiron not indicated for use in
children under 4 years of age) - Standard Schedule 1 dose per season, except 2
doses for children under 9 yrs of age in the
first season - Storage Refrigerate, 2-8C (35-46F)
72
Comparison of Influenza Vaccines
- Live, attenuated intranasal vaccine (LAIV) Dose
0.25ml in each nostril - Route Intranasal
- Labeled Age Range 2 (approved 9/19/07) to 49
years healthy!! - Standard Schedule 1 dose per season, except 2
doses for children under 9 yrs of age in the
first season - New storage guidelines
73
Inactivated Influenza Vaccine Efficacy
- 70-90 effective among healthy persons lt65 years
of age - 30-40 effective among frail elderly persons
- 50-60 effective in preventing hospitalization
- 80 effective in preventing death
74
Inactivated Influenza Vaccine Recommendations
- All persons 50 years of age or older
- Children 6-23 months of age
- Residents of long-term care facilities
- Pregnant women
- Persons 6 months to 18 years receiving chronic
aspirin therapy - Persons gt6 months of age with chronic illness
75
Inactivated Influenza Vaccine Recommendations
- Persons with the following chronic illnesses
should be considered for inactivated influenza
vaccine - pulmonary (e.g., asthma, COPD)
- cardiovascular (e.g., CHF)
- metabolic (e.g., diabetes)
- renal dysfunction
- hemoglobinopathy
- immunosuppression, including HIV infection
- any condition that can compromise respiratory
function or the handling of respiratory
secretions
76
Inactivated Influenza Vaccine Adverse Reactions
- Local reactions 15-20
- Fever, malaise not common
- Allergic reactions rare
- Neurological very rare reactions
77
Inactivated Influenza VaccineContraindications
and Precautions
- SEVERE allergic reaction to a vaccine component
(e.g., egg) or following a prior dose of vaccine - Influenza vaccines are produced in hen eggs
- - If a person can tolerate eating eggs,
vaccination is recommended - - If eating eggs causes anaphylaxis or laryngeal
edema, refer to an allergist or primary care
physician - Moderate or severe acute illness
78
Special considerations
- Patients who are pregnant and patients with HIV
are highly susceptible to complications from
influenza and are therefore good candidates for
vaccination with TIV but not LAIV
79
Influenza Vaccine Recommendations
- Healthcare providers, including home care
- Employees of long-term care facilities
- Household contacts of high-risk persons
LAIV should not be administered to healthcare
workers who have contact with severely
immunosuppressed persons who require
hospitalization and care in a protective
environment
80
Influenza Vaccine Recommendations
- Providers of essential community services
- Students
- Persons traveling outside the U.S.
- Anyone who wishes to reduce the likelihood of
becoming ill from influenza
these groups may receive TIV, and some may be
eligible for LAIV
81
Live Attenuated Influenza Vaccine Indications
(FluMist)
- Healthy persons 2-49 years of age
- close contacts of persons at high risk for
complications of influenza (except
immunosuppressed) - persons who wish to reduce their own risk of
influenza
Persons who do not have medical conditions that
increase their risk for complications of influenza
82
Live Attenuated Influenza VaccineContraindication
s and Precautions
- Children lt2 years of age
- Persons gt50 years of age
- Persons with chronic medical conditions
- Children and adolescents receiving long-term
aspirin therapy
These persons should receive inactivated
influenza vaccine
83
Live Attenuated Influenza VaccineContraindication
s and Precautions
- Immunosuppression from any cause
- Pregnant women
- Severe (anaphylactic) allergy to egg or other
vaccine components - History of Guillian-Barré syndrome
- Moderate or severe acute illness
These persons should receive inactivated
influenza vaccine
84
Live Attenuated Influenza VaccineAdverse
Reactions
- Children
- no significant increase in URI symptoms, fever,
or other systemic symptoms - increased risk of asthma or reactive airways
disease in children 12-59 months of age - Adults
- significantly increased rate of cough, runny
nose, nasal congestion, sore throat, and chills
reported among vaccine recipients - no increase in the occurrence of fever
- No serious adverse reactions identified
85
LAIV Storage and Handling New guidelines for
2007-08
- Shipped frozen
- Store in refrigerator between 2-8C (35-46F)
upon receipt and until use before the expiration
date - Should not be refrozen after thawing
86
Pneumococcal PneumoniaClinical Features
- Abrupt onset
- Fever
- Shaking chills
- Pleuritic chest pain
- Productive cough
- Dyspnea, tachypnea, hypoxia
87
Pneumococcal Pneumonia
- Estimated 175,000 hospitalizations per year in
the United States - Up to 36 of adult community-acquired pneumonia
and 50 of hospital-acquired pneumonia - Common bacterial complication of influenza and
measles - Case-fatality rate 5-7, higher in elderly
88
Burden of Pneumococcal Disease in Children
Syndrome Cases
- Bacteremia 13,000
- Meningitis 700
- Death 200
- Otitis media 5,000,000
Prior to routine use of pneumococcal conjugate
vaccine
89
Pneumococcal Disease Epidemiology
- Reservoir Human carriers
- Transmission Respiratory
Autoinoculation - Temporal pattern Winter and early spring
- Communicability Unknown
Probably as long as organism in respiratory
secretions
90
Pneumovax Prevnar Comparison
- Pneumovax Prevnar
- Serotype Valence 23-valent 7-valent
- Packaging 0.5ml syringes 0.5ml vials
- 0.5 0.25 ml vials
- Dosage Form Solution Suspension
- Storage Refrigeration Refrigeration
- Indication Everyone gt65 yrs Children lt 2 yrs
- pts with some chronic diseases kids with
invasive disease lt 5 yrs. - Dose 0.5ml once or twice in a lifetime 0.5ml at
2, 4, 6 12-15 months - Older children receive 1-3 doses
- depending on age.
91
Pneumococcal Polysaccharide Vaccine
- Purified pneumococcal polysaccharide (23 types)
- Not effective in children lt2 years
- 60-70 against invasive disease
- Less effective in preventing pneumococcal
pneumonia
92
Pneumococcal Polysaccharide Vaccine
Recommendations
- Adults gt65 years of age
- Persons gt2 years with
- chronic illness
- anatomic or functional asplenia
- immunocompromised (disease, chemotherapy,
steroids) - HIV infection
- environments or settings with increased risk
93
Pneumococcal Conjugate Vaccine (Prevnar)
- Pneumococcal polysaccharide conjugated to
nontoxic diphtheria toxin (7 serotypes) - Vaccine serotypes account for 86 of bacteremia
and 83 of meningitis among children lt6 years of
age
94
Pneumococcal Vaccines Adverse Reactions
- Local reactions
- polysaccharide 30-50
- conjugate 10-20
- Fever, myalgia
- polysaccharide lt1
- conjugate 15-24
- Severe adverse rarereactions
95
Pneumococcal Polysaccharide VaccineMissed
Opportunities
- gt65 of patients with severe pneumococcal disease
had been hospitalized within preceding 3-5 years
yet few had received vaccine - May be administered simultaneously with influenza
vaccine
96
Meningococcal Disease Clinical Features
- Incubation period 3-4 days (range 2-10 days)
- Abrupt onset of fever, meningeal symptoms,
hypotension, and rash - Fatality rate 9-12 up to 40 in
meningococcemia - Two primary clinical presentations
- Systemic infection via the blood (septicemia or
meningococcemia) - Infection of the fluid of the spinal cord and
brain (meningitis).
97
Meningococcal Disease Epidemiology
- Reservoir Human
- Transmission Respiratory droplets
- Temporal pattern Peaks in late winterearly
spring - Communicability Generally limited
98
Meningococcal Disease United States
- Adolescents and young adults account for nearly
30 percent of all cases of meningitis in the
United States. - 100 to 125 cases of meningococcal disease occur
on college campuses each year. - 5-15 students die as a result annually.
- Evidence shows approximately 70 to 80 percent of
cases in the college age group are caused by
serogroup C, Y, or W-135, which are potentially
vaccine-preventable.
99
CDC Recommendations for Immunization of College
Students in Dorms
- On February 10, 2005, the Advisory Committee on
Immunization Practices (ACIP) for the Centers for
Disease Control and Prevention (CDC) voted to
recommend that all incoming college freshmen
living in dormitories or residence halls be
vaccinated against meningococcal disease. - The ACIP also recommended vaccination for all
adolescents at high school entry and during
pre-adolescent health care visits (11 to 12 years
old).
100
Meningococcal Polysaccharide Vaccine (MPSV)
- Menomune (sanofi pasteur)
- Quadrivalent polysaccharide vaccine (A, C, Y,
W-135) - Administered by subcutaneous injection
- 10-dose vial contains thimerosal as a preservative
101
MPSV Recommendations
- Approved for persons gt2 years of age
- Not recommended for routine vaccination of
civilians - Should be used only for persons at increased risk
of N. meningiditis infection who are 2-10 years
or gt55 years of age, or if MCV is not available
102
Meningococcal Conjugate Vaccine (MCV)
- Menactra (Sanofi pasteur)
- Quadrivalent polysaccharide vaccine (A, C, Y,
W-135) conjugated to diphtheria toxoid - Administered by intramuscular injection
- Single dose vials do not contain a preservative
103
MCV Recommendations (revised as of June 2007)
- Routinely recommended for
- All children at 11-18 years of age
- Other persons 19-55 years of age at increased
risk of invasive meningococcal disease
MMWR 2005 54(RR-7)1-21
104
Meningococcal VaccinesAdverse Reactions
MPSV - Menomune
MCV- Menactra
- Local reactions 4-48 11-59
- for 1-2 days
- Fever gt100oF 3
5 - Systemic reactions 3-60 4-62
- (headache, malaise
- fatigue)
105
Meningococcal VaccineRecommendations
- Use of MCV is preferred for persons 11-55 years
of age for whom meningococcal vaccine is
recommended - MPSV should be used for persons 2-10 years and
gt55 years - Use of MPSV is an acceptable alternative for
persons 11-55 years of age if MCV is not
available
MMWR 2005 54(RR-7)1-21
106
Bioterrorism Information
- CDC Emergency Preparedness and Response Website
- www.bt.cdc.gov
- Anthrax vaccine
- Smallpox vaccine
107
Vaccines on the horizon
- Hepatitis C
- Staphylococcus aureus
- Cytomegalovirus
- Group B beta-hemolytic strep
- Tuberculosis
- Malaria