Arterial Plaque and - PowerPoint PPT Presentation
1 / 147
Title: Arterial Plaque and
1
(No Transcript)
2
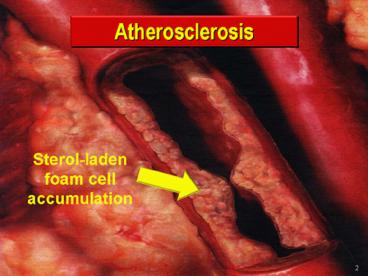
Arterial Plaque and Thrombus Formation by Neville
Woolf and Michael J. Davies
3
(No Transcript)
4
(No Transcript)
5
Atherosclerosis -- An Inflammatory Disease
6
(No Transcript)
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
(No Transcript)
11
(No Transcript)
12
(No Transcript)
13
(No Transcript)
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
(No Transcript)
18
(No Transcript)
19
(No Transcript)
20
(No Transcript)
21
(No Transcript)
22
(No Transcript)
23
(No Transcript)
24
(No Transcript)
25
(No Transcript)
26
(No Transcript)
27
(No Transcript)
28
(No Transcript)
29
(No Transcript)
30
(No Transcript)
31
(No Transcript)
32
(No Transcript)
33
(No Transcript)
34
(No Transcript)
35
(No Transcript)
36
(No Transcript)
37
(No Transcript)
38
(No Transcript)
39
(No Transcript)
40
(No Transcript)
41
(No Transcript)
42
(No Transcript)
43
(No Transcript)
44
The Collaborative AtoRvastatin Diabetes Study
(CARDS)Rationale and Study Design
CARDS March 2004
45
The Collaborative AtoRvastatin Diabetes Study
(CARDS) Design
46
CARDS Overview
- CARDS is the first primary prevention statin
trial conducted solely in patients with type 2
diabetes - The entry criteria were chosen to define a group
of patients with type 2 diabetes at increased
risk for CV morbidity and mortality, but without
established clinical CVD - 2838 patients with diabetes and at least 1
otherCVD risk factor - Multicenter, randomized, placebo-controlled,
double-blind trial of atorvastatin 10 mg/day - Patient recruitment from 132 clinical centers in
the UK and Ireland - The anticipated study period was 4 years
however, the trial was stopped early due to a
beneficial effect in atorvastatin-treated patients
Colhoun HM, Thomason MJ, Mackness MI, et al.
Diabet Med. 200219201-211.
47
Primary Efficacy Analysis
- Time from randomization to the first occurrence
of a primary endpoint in the two treatment arms - Primary endpoint is a composite endpoint
- Major coronary event (fatal or non-fatal MI,
other CHD death) - Silent MI
- Stroke
- Resuscitated cardiac arrest
- Coronary revascularization procedure
- Unstable angina requiring hospitalization
48
Trial Stopped Early Due to Significant Benefit of
Atorvastatin Treatment
- In June 2003, the independent steering committee
stopped the trial early because the magnitude of
benefit for the primary end point exceeded the
prespecified stopping rule - Preliminary results of the CARDS trial showed a
significant reduction in MI, stroke, and other
coronary events in patients treated with
atorvastatin - CARDS became the second atorvastatin trial to end
early because of observed treatment benefit
(ASCOT-LLA was the first)
http//www.pfizer.com/download/news/2003Q2_earnqa
49
(No Transcript)
50
(No Transcript)
51
(No Transcript)
52
(No Transcript)
53
(No Transcript)
54
(No Transcript)
55
(No Transcript)
56
(No Transcript)
57
ELIGIBILITY MRC/BHF Heart Protection Study
- Increased risk of CHD death due to prior disease
- Myocardial infarction or other coronary heart
disease - Occlusive disease of non-coronary arteries or
- Diabetes mellitus or treated hypertension
- Age 40-80 years
- Total cholesterol gt3.5 mmol/l (gt135mg/dl)
- Statin or vitamins not considered clearly
indicated or contraindicated by patients own
doctors
58
FACTORIAL TREATMENT COMPARISONS
59
(No Transcript)
60
(No Transcript)
61
Average - 0.96 0.02 mmol/l - 37 0.8
mg/dl
62
(No Transcript)
63
(No Transcript)
64
(No Transcript)
65
(No Transcript)
66
(No Transcript)
67
(No Transcript)
68
(No Transcript)
69
(No Transcript)
70
SIMVASTATIN Safety monitoring
71
SIMVASTATIN Main conclusions
- After allowance for non-compliance, 40mg daily
simvastatin safely reduces the risk of heart
attack, of stroke, and of revascularisation by at
least one-third - 5 years of statin treatment typically prevents
these major vascular events in about - 100 of every 1000 with previous MI
- 80 " " other CHD
- 70 " " diabetes (age 40)
- 70 " " previous stroke
- 70 " " other PVD
irrespective of cholesterol level (or age, or
sex, or other treatments)
72
(No Transcript)
73
(No Transcript)
74
(No Transcript)
75
(No Transcript)
76
(No Transcript)
77
(No Transcript)
78
(No Transcript)
79
(No Transcript)
80
(No Transcript)
81
(No Transcript)
82
(No Transcript)
83
(No Transcript)
84
(No Transcript)
85
(No Transcript)
86
HDL metabolism andreverse cholesterol transport
VLDL
TG
CETP
LDL
HDL
Hepatic catabolism
Cla-1/SR-BI receptor
Apo A-II
Liver
Pre b-HDL
Hepatic synthesis
Apo A-I
Free cholesterol
LPL
Chylomicron remnant
Intestine
ABCA-1 transporter
Chylomicron
Intestinal synthesis
Peripheral cell
87
Vol. 298 No. 7, August 15, 2007
Review
CLINICIAN'S CORNER
High-Density Lipoprotein as a Therapeutic Target
88
(No Transcript)
89
(No Transcript)
90
(No Transcript)
91
(No Transcript)
92
(No Transcript)
93
(No Transcript)
94
(No Transcript)
95
(No Transcript)
96
(No Transcript)
97
(No Transcript)
98
(No Transcript)
99
(No Transcript)
100
(No Transcript)
101
(No Transcript)
102
(No Transcript)
103
(No Transcript)
104
(No Transcript)
105
(No Transcript)
106
(No Transcript)
107
(No Transcript)
108
(No Transcript)
109
(No Transcript)
110
(No Transcript)
111
(No Transcript)
112
(No Transcript)
113
(No Transcript)
114
(No Transcript)
115
(No Transcript)
116
(No Transcript)
117
(No Transcript)
118
(No Transcript)
119
(No Transcript)
120
(No Transcript)
121
(No Transcript)
122
(No Transcript)
123
(No Transcript)
124
(No Transcript)
125
(No Transcript)
126
(No Transcript)
127
(No Transcript)
128
(No Transcript)
129
(No Transcript)
130
(No Transcript)
131
(No Transcript)
132
(No Transcript)
133
(No Transcript)
134
(No Transcript)
135
(No Transcript)
136
(No Transcript)
137
(No Transcript)
138
(No Transcript)
139
(No Transcript)
140
(No Transcript)
141
Lipid Management Goal
LDL-C should be less than 100 mg/dL Further
reduction to LDL-C to lt 70 mg/dL is reasonable
If TG gt200 mg/dL, non-HDL-C should be lt 130
mg/dL
Non-HDL-C total cholesterol minus HDL-C
142
Lipid Management Goals NCEP
ATPAdult Treatment Panel, CHDCoronary heart
disease, LDL-CLow-density lipoprotein
cholesterol, TLCTherapeutic lifestyle changes
Grundy, S. et al. Circulation 2004110227-39.
143
Lipid Management Recommendations
For all patients
Start dietary therapy (lt7 of total calories as
saturated fat and lt200 mg/d cholesterol) Adding
plant stanol/sterols (2 gm/day) and viscous fiber
(gt10 mg/day) will further lower LDL Promote
daily physical activity and weight management.
Encourage increased consumption of omega-3
fatty acids in fish or 1 g/day omega-3 fatty
acids in capsule form for risk reduction.
144
ATP III Dietary Recommendations
ATPAdult Treatment Panel
Expert Panel on Detection, Evaluation, and
Treatment of High Blood Cholesterol in Adults.
JAMA 20012852486-2497.
145
Lipid Management Recommendations
Assess fasting lipid profile in all patients, and
within 24 hours of hospitalization for those with
an acute event. For patients hospitalized,
initiate lipid-lowering medication as recommended
below prior to discharge according to the
following schedule
If baseline LDL-C gt 100 mg/dL, initiate
LDL-lowering drug therapy If on-treatment LDL-C gt
100 mg/dL, intensify LDL-lowering drug therapy
(may require LDL lowering drug combination) If
baseline is LDL-C 70 to 100 mg/dL, it is
reasonable to treat to LDL lt 70 mg/dL
When LDL lowering medications are used, obtain at
least a 30-40 reduction in LDL-C levels.
146
Lipid Management Recommendations
If TG are 200-499 mg/dL, non-HDL-C should be lt
130 mg/dL Further reduction of non-HDL to lt 100
mg/dL is reasonable Therapeutic options to
reduce non-HDL-C More intense LDL-C lowering
therapy I (B) or Niacin (after LDL-C lowering
therapy) IIa (B) or Fibrate (after LDL-C lowering
therapy) IIa (B) If TG are gt 500 mg/dL,
therapeutic options to prevent pancreatitis are
fibrate or niacin before LDL lowering therapy
and treat LDL-C to goal after TG-lowering
therapy. Achieve non-HDL-C lt 130 mg/dL, if
possible
147
(No Transcript)
148
Implications of Recent Clinical Trials for
the National Cholesterol Education Program Adult
Treatment Panel III Guidelines
Scott M. Grundy James I. Cleeman C. Noel Bairey
Merz H. Bryan Brewer, Jr Luther T.
Clark Donald B. Hunninghake Richard C.
Pasternak Sidney C. Smith, Jr Neil J.
Stone for the Coordinating Committee of the
National Cholesterol Education Program
149
TABLE 3. Recommendations for Modifications to
Footnote the ATP III Treatment Algorithm for
LDL-C ? Therapeutic lifestyle changes (TLC)
remain an essential modality in clinical
management. TLC has the potential to reduce
cardiovascular risk through several mechanisms
beyond LDL lowering. ? In high-risk persons, the
recommended LDL-C goal is 100 mg/dL. An LDL-C
goal of 70 mg/dL is a therapeutic option on the
basis of available clinical trial evidence,
especially for patients at very high risk. If
LDL-C is 100 mg/dL, an LDL-lowering drug is
indicated simultaneously with lifestyle changes.
150
Gracias
151
Gracias