The%20Mole - PowerPoint PPT Presentation
Title:
The%20Mole
Description:
... atomic mass (in grams) of a mole of atoms of the element. ... This means 1 mole H2O = 18.0 g H2O. Therefore, we can turn this into two conversion factors. ... – PowerPoint PPT presentation
Number of Views:1507
Avg rating:3.0/5.0
Title: The%20Mole
1
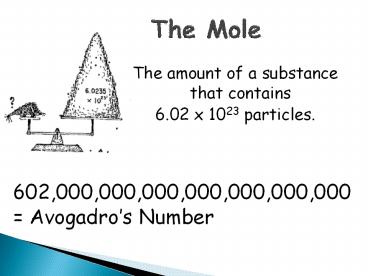
The Mole
- The amount of a substance that contains
- 6.02 x 1023 particles.
- 602,000,000,000,000,000,000,000
- Avogadros Number
2
The Mole
- The abbreviation for mole is
mol
3
Molar Mass
- Molar mass of an element mass in grams (g) of a
mole of atoms of that element. - Ex. C 12.0 g, H 1.0 g, O 16.0 g
- You can read this on your periodic table. It
represents the average atomic mass (in grams) of
a mole of atoms of the element.
4
Molar Mass of a Compound
- Simply requires basic multiplication and
addition. - Ex. What is the molar mass of water, H2O?
- 1 mole of water has 2 moles of hydrogen and 1
mole of oxygen. - 2 mole ? H at 1.0 g/mol 2.0 g H
- 1 mole ? O at 16.0 g/mol 16.0 g O
- 2.0 g 16.0 g 18.0 g/mol
5
Find the molar mass of Al2(SO4)3
- 1 mole of Al2(SO4)3 has 2 moles of aluminum, 3
moles of sulfur, and 12 moles of oxygen. - Molar mass of Al 27.0 g/mol
- Molar mass of S 32.1 g/mol
- Molar mass of O 16.0 g/mol
- 2 x (27.0 g/mol) 3 x (32.1 g/mol) 12 x (16.0
g/mol) - 342.3 g/mol
6
Molar Mass as a Conversion Factor
- The molar mass of H2O 18.0 g/mol
- This means 1 mole H2O 18.0 g H2O
- Therefore, we can turn this into two conversion
factors. - 18.0 g 1 mole
- 1 mole 18.0 g
7
Mole Conversions
- moles ? grams Use Molar Mass
- grams ? moles
- Moles ? grams
- Ex. What is the mass of 3.0 moles Carbon?
8
Mole Conversions (moles ? grams)
- Ex. What is the mass of 3.0 moles Carbon?
- One mole of carbon weighs 12.0 g
- Three moles of carbon weighs 3 x 12.0 g
- 36.0 g
9
Mole Conversions (moles ? grams)
What is the mass of 4.5 moles of H2O?
One mole of H2O 1.0g (H) 1.0g (H) 16.0g
(O) 18.0g
4.5 moles of H2O 4.5 x 18.0g 81.0g
10
Mole Conversions (grams ? moles)
How many moles is 44.5 g H2O?
One mole of H2O 1.0g (H) 1.0g (H) 16.0g
(O) 18.0g/mol
44.5g 18.0g/mol
2.47 mol H2O
11
Mole Conversions (grams ? moles)
How many moles is 2.5 g NaCl?
One mole of NaCl 22.99g (Na) 35.45g (Cl)
58.44g/mol
2 sig. digs.
2.5g 58.44g/mol
.04 mol NaCl
12
molecules/atoms ? molesmoles ? molecules/atoms
- Use Avogadros
- 6.02 x 1023 moles/atoms
- mole
- Ex. Molecules ? moles
- How many moles is 9.00 x 1023 molecules of CO2?
13
molecules/atoms ? moles
- How many moles is 9.00 x 1023 molecules of CO2?
9.00 x 1023 molecules 6.02 x 1023 molecules
1.50 mole of CO2
14
Moles ? molecules/atoms How many molecules is
1.75 moles H2O?
1.75mole x 6.02 x 1023 atoms x 1 molecule H2O
mole 3 atoms
3.51 x 1023 molecules H2O
15
Combining Steps
16
How many molecules is 50.0 g H2O? What is the
mass of 9.0 x 1023 molecules of CO2?