Electrochemistry - PowerPoint PPT Presentation
Title:
Electrochemistry
Description:
Each of the above reactions is called a half reaction. ... Step 4) Add the two half-reactions and simplify where possible by canceling ... – PowerPoint PPT presentation
Number of Views:896
Avg rating:3.0/5.0
Title: Electrochemistry
1
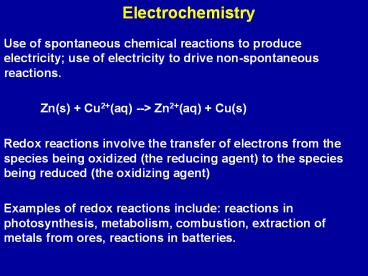
Electrochemistry
- Use of spontaneous chemical reactions to produce
electricity use of electricity to drive
non-spontaneous reactions. - Zn(s) Cu2(aq) -- Zn2(aq) Cu(s)
- Redox reactions involve the transfer of electrons
from the species being oxidized (the reducing
agent) to the species being reduced (the
oxidizing agent) - Examples of redox reactions include reactions in
photosynthesis, metabolism, combustion,
extraction of metals from ores, reactions in
batteries.
2
The transfer of electrons in the reaction
Zn(s) Cu2(aq) -- Zn2(aq) Cu(s) is
thermodynamically favorable the reaction
proceeds spontaneously. DGfo Zn2(aq) -147.06
kJ/mol DGfo Cu2(aq) 65.49 kJ/mol DGro
-212.55 kJ/mol
The transfer of electrons can used to produce
energy in the form of electricity - chemical
energy converted to electrical energy.
3
- Balancing Oxidation-Reduction Reactions
- Sn2(aq) Fe3 -- Sn4(aq) Fe2(aq)
- This reaction is balanced in terms of mass but
not charge. - As written, the oxidation process (Sn2 to Sn4)
involves two electrons and the reduction process
(Fe3 to Fe2) one electron - To balance redox reactions it is easier to
consider the oxidation and reduction reactions
separately, even though one cannot occur without
the other.
4
Oxidation Sn2(aq) -- Sn4(aq)
2e- Reduction Fe3(aq) e- -- Fe2(aq) Each
of the above reactions is called a half
reaction. In writing half reactions, the number
of electrons lost in the oxidation half reaction
must equal the number of electrons gained in the
reduction half reaction Oxidation Sn2(aq) --
Sn4(aq) 2e- Reduction 2Fe3(aq) 2e- --
2Fe2(aq)
5
The overall reaction is then the sum of the two
half reactions Sn2(aq) 2Fe3 -- Sn4(aq)
2Fe2(aq) Writing the half reactions so that
the number of electrons lost in the oxidation
half reaction equals the number of electrons
gained in the reduction half reaction ensures
that the overall reaction is balanced in terms of
charge.
6
- Systematic procedure for balancing redox
reactions - The unbalanced redox reaction between MnO4- and
C2O42- reaction in an acidic aqueous solution is - MnO4-(aq) C2O42- (aq) -- Mn2(aq) CO2 (g)
- Step 1) Divide the equation into two incomplete
half-reactions, one for oxidation and one for
reduction
7
- Step 2) Balance each half reaction
- a) First balance the elements other than H and O
- MnO4-(aq) -- Mn2(aq)
- C2O42- (aq) -- 2CO2 (g)
- b) Next balance the O atoms by adding H2O
- MnO4-(aq) -- Mn2(aq) 4H2O(l)
- C2O42- (aq) -- 2CO2 (g)
- c) Balance the H atoms for acidic solution
balance H by adding H, for basic solutions
balance H by adding H2O to the side deficient in
H and an equal amount of OH- to the other side. - MnO4-(aq) 8 H(aq) -- Mn2(aq) 4H2O(l)
- C2O42- (aq) -- 2CO2 (g)
8
- d) Balance the charge by adding electrons (e-) to
the side with the greater overall positive
charge, so that the sum of the charge on the left
sum of charge on the right - MnO4-(aq) 8 H(aq) 5e- -- Mn2(aq)
4H2O(l) - C2O42- (aq) -- 2CO2 (g) 2e-
- Step 3) Multiply each half reaction by an integer
so that the number of electrons lost in one half
reaction equals the number gained in the other. - MnO4-(aq) 8 H(aq) 5e- -- Mn2(aq)
4H2O(l) - C2O42- (aq) -- 2CO2 (g) 2e-
- Multiply the by MnO4- reaction 2 and the C2O42-
reaction by 5 to balance charge - 2MnO4-(aq) 16 H(aq) 10e- -- 2Mn2(aq)
8H2O(l) - 5C2O42- (aq) -- 10CO2 (g) 10e-
9
- Step 4) Add the two half-reactions and simplify
where possible by canceling species appearing on
both sides of the equation.
2MnO4-(aq) 16 H(aq) 5C2O42- (aq) 10e- --
2Mn2(aq) 8H2O(l) 10CO2 (g) 10e-
2MnO4-(aq) 16 H(aq) 5C2O42- (aq) --
2Mn2(aq) 8H2O(l) 10CO2 (g)
Step 5) Check the equation for mass and charge
balance
10
- Balance the following reaction which takes place
in a basic solution
Ag(s) HS-(aq) CrO42-(aq) -- Ag2S(s)
Cr(OH)3(s)
Step 1) the two half reactions are Ag(s)
HS-(aq) -- Ag2S(s) CrO42-(aq) --
Cr(OH)3(s) Step 2a) balance elements other than
H and O 2 Ag(s) HS-(aq) -- Ag2S(s)
CrO42-(aq) -- Cr(OH)3(s)
11
- Step 2b) Balance O
- 2Ag(s) HS-(aq) -- Ag2S(s)
- CrO42-(aq) -- Cr(OH)3(s) H2O(l)
- Step 2c) Balance H this is a basic solution.
For basic solutions balance H by adding H2O to
the side deficient in H and an equal amount of
OH- to the other side. - 2Ag(s) HS-(aq) OH- -- Ag2S(s) H2O(l)
- CrO42-(aq) 5H2O(l) -- Cr(OH)3(s) H2O(l)
5OH-(aq) - CrO42-(aq) 4H2O(l) -- Cr(OH)3(s) 5OH- (aq)
- Step 2d) Balance charge in each half reaction
- 2Ag(s) HS-(aq) OH- -- Ag2S(s) H2O(l) 2e-
- CrO42-(aq) 4H2O(l) 3e- -- Cr(OH)3(s) 5OH-
(aq)
12
- Step 3) Multiply each half reaction by integers
to balance charge between the two half reactions - 6Ag(s) 3HS-(aq) 3OH- -- 3Ag2S(s) 3H2O(l)
6e- - 2CrO42-(aq) 8H2O(l) 6e- -- 2Cr(OH)3(s)
10OH- (aq) - Step 4) Add half reactions, accounting for
species that appear on both sides. - 6Ag(s) 3HS-(aq) 3OH- 2CrO42-(aq) 8H2O(l)
6e- -- 3Ag2S(s) 3H2O(l) 6e-
2Cr(OH)3(s) 10OH- (aq) - 6Ag(s) 3HS-(aq) 2CrO42-(aq) 5 H2O(l) --
3Ag2S(s) 2Cr(OH)3(s)
7OH- (aq) - Step 5) Check for charge and mass balance
13
Balancing Disproportionation Reactions In a
disproportionation reaction, the same species is
both oxidized and reduced. To balance such
reactions, realize that the same species may
appear on the left of BOTH half
reactions. Example Balance the reaction below
which takes place in an acidic solution Cl2(aq)
-- ClO3-(aq) Cl-(aq) Step 1 Cl2 -- ClO3-
Cl2 -- Cl-
14
Balancing O and H and charges in each half
reaction Cl2 6H2O -- 2ClO3- 12H
10e- (oxidation) Cl2 2e- -- 2Cl-
(reduction) Accounting for the different charges
in each half reaction - multiply reduction
reaction by 5. The add the two half
reactions 6Cl2 6H2O -- 2ClO3- 10Cl- 12H
Divide through by 2 3Cl2(aq) 3H2O(l) --
ClO3-(aq) 5Cl-(aq) 6H(aq)
15
- Electrochemical Cells
- The energy released in a spontaneous redox
reaction can be used to perform electrical work. - This is accomplished through a voltaic or
galvanic cell, a device in which the transfer of
electrons takes place through an external pathway
rather than directly between reactants. - Consider the spontaneous reaction that occurs
when a strip of Zn is placed in a solution
containing Cu2. - The reaction taking place is
- Zn(s) Cu2(aq) -- Cu(s) Zn2(aq)
16
- Zn(s) Cu2(aq) -- Cu(s) Zn2(aq)
- As the reaction proceeds the blue color of the
Cu2 fades and Cu(s) is deposited on the Zn strip - The Zn strip is in direct contact with the Cu2
and the exchange of electrons between Zn and Cu2
occur directly between reactants.
17
The Zn and Cu2 are no longer in direct
contact. The reduction of the Cu2 can occur
only by a flow of electrons through an external
circuit, namely the wire connecting the Zn(s) and
Cu(s) strips the Zn and Cu strips are the
ELECTRODES.
18
- The voltaic cell can be considered to be made up
of two half-cells. In each half cell a
half-reaction takes place.
19
In the solution containing the Zn strip the
following half reaction takes place Zn(s) --
Zn2(aq) 2 e- Oxidation In the solution
containing the Cu strip the following half
reaction takes place Cu2(aq) 2 e- --
Cu(s) Reduction
20
- The electrode at which oxidation occurs is called
the ANODE here Zn is the anode. The anode is
negative. - The electrode at which reduction occurs is called
the CATHODE here Cu is the cathode. The
cathode is positive - As the Zn metal is oxidized, the electrons flow
from the anode (-) through the external circuit
to the cathode () where they are taken up in the
reduction of Cu2 to Cu(s)
21
As the oxidation-reduction reactions are
proceeding, since there is charge transfer
between the two solutions, one side will become
more positive and the other more negative,
preventing charge flow between the two cells. To
prevent charge buildup, a salt bridge is setup
between the two cells.
22
- The salt bridge consists of a solution of an
electrolyte like NaNO3 whose ions will not react
with the ions in the cell. - As oxidation and reduction takes place, the ions
in the salt bridge migrate in the directions
required to maintain charge neutrality.