Redox Titrations - PowerPoint PPT Presentation
1 / 30
Title:
Redox Titrations
Description:
Many common analytes in chemistry, biology, environmental and materials science ... b) Jones reductor (Zn Zn amalgam anything in mercury) Redox Titrations ... – PowerPoint PPT presentation
Number of Views:2059
Avg rating:3.0/5.0
Title: Redox Titrations
1
Redox Titrations
- Introduction
- 1.) Redox Titration
- Based on an oxidation-reduction reaction between
analyte and titrant - Many common analytes in chemistry, biology,
environmental and materials science can be
measured by redox titrations
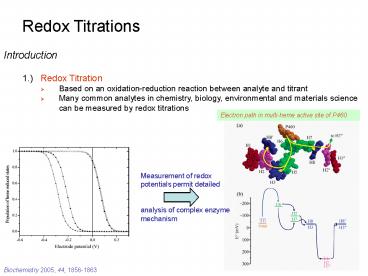
Electron path in multi-heme active site of P460
Measurement of redox potentials permit detailed
analysis of complex enzyme mechanism
Biochemistry 2005, 44, 1856-1863
2
Redox Titrations
- Shape of a Redox Titration Curve
- 1.) Voltage Change as a Function of Added Titrant
- Consider the Titration Reaction (essentially goes
to completion) - Ce4 is added with a buret to a solution of Fe2
- Pt electrode responds to relative concentration
- of Fe3/Fe2 Ce4/Ce3
- Calomel electrode used as reference
K 1016
Indicator half-reactions at Pt electrode
Eo 0.767 V
Eo 1.70 V
3
Redox Titrations
- Shape of a Redox Titration Curve
- 2.) Titration Curve has Three Regions
- Before the Equivalence Point
- At the Equivalence Point
- After the Equivalence Point
- 3.) Region 1 Before the Equivalence Point
- Each aliquot of Ce4 creates an equal
- number of moles of Ce3 and Fe3
- Excess unreacted Fe2 remains in solution
- Amounts of Fe2 and Fe3 are known, use
- to determine cell voltage.
- Residual amount of Ce4 is unknown
4
Redox Titrations
- Shape of a Redox Titration Curve
- 3.) Region 1 Before the Equivalence Point
Use iron half-reaction relative to calomel
reference electrode
Eo 0.767 V
Potential of calomel electrode
Simplify
5
Redox Titrations
- Shape of a Redox Titration Curve
- 3.) Region 1 Before the Equivalence Point
- Special point when V 1/2 Ve
Log term is zero
The point at which V ½ Ve is analogous to the
point at which pH pKa in an acid base titration
6
Redox Titrations
- Shape of a Redox Titration Curve
- 3.) Region 1 Before the Equivalence Point
- Another special point, when Ce40
- Voltage can not be calculated
- Fe3 is unknown
- If Fe3 0, Voltage -8
- Must be some Fe3 from impurity
- or Fe2 oxidation
- Voltage can never be lower than value need
- to reduce the solvent
Eo -0.828 V
7
Redox Titrations
- Shape of a Redox Titration Curve
- 3.) Region 1 Before the Equivalence Point
- Special point when V 2Ve
Log term is zero
The point at which V 2 Ve is analogous to the
point at which pH pKa in an acid base titration
8
Redox Titrations
- Shape of a Redox Titration Curve
- 4.) Region 2 At the Equivalence Point
- Enough Ce4 has been added to react with all Fe2
- Primarily only Ce3 and Fe3 present
- Tiny amounts of Ce4 and Fe2 from equilibrium
- From Reaction
- Ce3 Fe3
- Ce4 Fe2
- Both Reactions are in Equilibrium at the
- Pt electrode
9
Redox Titrations
- Shape of a Redox Titration Curve
- 4.) Region 2 At the Equivalence Point
- Dont Know the Concentration of either Fe2 or
Ce4 - Cant solve either equation independently to
determine E - Instead Add both equations together
Add
Rearrange
10
Redox Titrations
- Shape of a Redox Titration Curve
- 4.) Region 2 At the Equivalence Point
- Instead Add both equations together
Log term is zero
Cell voltage
Equivalence-point voltage is independent of the
concentrations and volumes of the reactants
11
Redox Titrations
- Shape of a Redox Titration Curve
- 5.) Region 3 After the Equivalence Point
- Opposite Situation Compared to Before the
Equivalence Point - Equal number of moles of Ce3 and Fe3
- Excess unreacted Ce4 remains in solution
- Amounts of Ce3 and Ce4 are known, use
- to determine cell voltage.
- Residual amount of Fe2 is unknown
12
Redox Titrations
- Shape of a Redox Titration Curve
- 5.) Region 3 After the Equivalence Point
Use iron half-reaction relative to calomel
reference electrode
Eo 1.70 V
Potential of calomel electrode
Simplify
13
Redox Titrations
- Shape of a Redox Titration Curve
- 6.) Titration Only Depends on the Ratio of
Reactants - Independent on concentration and/or volume
- Same curve if diluted or concentrated by a factor
of 10
14
Redox Titrations
- Shape of a Redox Titration Curve
- 7.) Asymmetric Titration Curves
- Reaction Stoichiometry is not 11
- Equivalence point is not the center of the steep
part of the titration curve
Titration curve for 21 Stoichiometry
2/3 height
15
Redox Titrations
- Finding the End Point
- 1.) Indicators or Electrodes
- Similar to Acid-Base Titrations
- Electrochemical measurements (current or
potential) can be used to determine the endpoint
of a redox titration - Redox Indicator is a chemical compound that
undergoes a color change as it goes from its
oxidized form to its reduced form - Similar to acid-base indicators that change color
with a change in protonation state
16
Redox Titrations
- Finding the End Point
- 2.) Redox Indicators
- Color Change for a Redox Indicator occurs mostly
over the range - where Eo is the standard reduction potential for
the indicator - and n is the number of electrons involved in the
reduction
17
Redox Titrations
- Finding the End Point
- 2.) Redox Indicators
- Color Change for a Redox Indicator occurs over a
potential range - Illustration
- For Ferroin with Eo 1.147V, the range of color
change relative to SHE - Relative to SCE is
18
Redox Titrations
- Finding the End Point
- 2.) Redox Indicators
- In order to be useful in endpoint detection, a
redox indicators range of color change should
match the potential range expected at the end of
the titration.
Relative to calomel electrode (-0.241V)
19
Redox Titrations
- Common Redox Reagents
- 1.) Starch
- Commonly used as an indicator in redox titrations
involving iodine - Reacts with iodine to form an intensely blue
colored complex - Starch is not a redox indicator
- Does not undergo a change in redox potential
I6 bound in center of starch helix
Repeating unit
20
Redox Titrations
- Common Redox Reagents
- 2.) Adjustment of Analyte Oxidation State
- Before many compounds can be determined by Redox
Titrations, must be converted into a known
oxidation state - This step in the procedure is known as
prereduction or preoxidation - Reagents for prereduction or preoxidation must
- Totally convert analyte into desired form
- Be easy to remove from the reaction mixture
- Avoid interfering in the titration
- Examples
- Preoxidation
- Peroxydisulfate or persulfate (S2O82-) with Ag
catalyst
Powerful oxidants
Oxidizes Mn2, Ce3, Cr3, VO2 excess S2O82- and
Ag removed by boiling the solution
21
Redox Titrations
- Common Redox Reagents
- 2.) Adjustment of Analyte Oxidation State
- Examples
- Preoxidation
- Silver(II) oxide (AgO) in concentrated mineral
acids also yields Ag2 - excess removed by boiling
- Hydrogen peroxide (H2O2) is a good oxidant to use
in basic solutions - Oxidizes Co2, Fe2, Mn2
- Reduces Cr2O72-, MnO4-
- excess removed by boiling
- Prereduction
- Stannous chloride (SnCl2) in hot HCl
- Reduce Fe3 to Fe2
- excess removed by adding HgCl2
- b) Jones reductor (Zn Zn amalgam anything in
mercury)
22
Redox Titrations
- Common Redox Reagents
- 3.) Common Titrants for Oxidation Reactions
- Potassium Permanganate (KMnO4)
- Strong oxidant
- Own indicator
pH 1
Titration of VO2 with KMnO4
Eo 1.507 V
Violet colorless
pH neutral or alkaline
Eo 1.692 V
Violet brown
Before Near After Equivalence point
pH strolngly alkaline
Eo 0.56 V
Violet green
23
Redox Titrations
- Common Redox Reagents
- 3.) Common Titrants for Oxidation Reactions
- Potassium Permanganate (KMnO4)
- Application of KMnO4 in Redox Titrations
24
Redox Titrations
- Common Redox Reagents
- 3.) Common Titrants for Oxidation Reactions
- Cerium (IV) (Ce4)
- Commonly used in place of KMnO4
- Works best in acidic solution
- Can be used in most applications in previous
table - Used to analyze some organic compounds
- Color change not distinct to be its own indicator
Yellow
colorless
Ce4 binds anions very strongly results in
variation of formal potential 1.70V in 1 F
HClO4 1.61V in 1 F HNO3 1.47V in 1 F
HCl 1.44V in 1 F H2SO4
Measure activity not concentration
Formal potential
25
Redox Titrations
- Common Redox Reagents
- 3.) Common Titrants for Oxidation Reactions
- Potassium Dichromate (K2Cr2O7)
- Powerful oxidant in strong acid
- Not as Strong as KMnO4 or Ce4
- Primarily used for the determination of Fe2
- Not an oxidant in basic solution
- Color change not distinct to be its own indicator
Eo 1.36 V
orange
green to violet
26
Redox Titrations
- Common Redox Reagents
- 3.) Common Titrants for Oxidation Reactions
- Iodine (Solution of I2 I-)
- I3- is actual species used in titrations with
iodine - Either starch of Sodium Thiosulfate (Na2S2O3) are
used as indicator
K 7 x 102
I3- Starch
I3-
I3- S2O32-
Before endpoint
Before endpoint
At endpoint
27
Redox Titrations
- Common Redox Reagents
- 3.) Common Titrants for Oxidation Reactions
- Iodine (Solution of I2 I-)
- Application of Iodine in Redox Titrations
28
Redox Titrations
- Common Redox Reagents
- 3.) Common Titrants for Oxidation Reactions
- Iodine (Solution of I2 I-)
- Application for Redox Titrations that Produce I3-
29
Redox Titrations
- Common Redox Reagents
- 3.) Common Titrants for Oxidation Reactions
- Periodic Acid (HIO4)
- Commonly used in titration of organic compounds
(especially carbohydrates) - 4.) Titrations with Reducing Agents
- Not as common as titrations using oxidizing
agents - Available titrants are not very stable in the
presence of atmospheric O2 - Reagents can be generated directly in solution by
means of chemical or electrochemical reactions
30
Redox Titrations
- Common Redox Reagents
- 5.) Example
- A 50.00 mL sample containing La3 was titrated
with sodium oxalate to precipitate La2(C2O4)3,
which was washed, dissolved in acid, and titrated
with 18.0 mL of 0.006363 M KMnO4. - Calculate the molarity of La3 in the unknown.