Visible Spectrum - PowerPoint PPT Presentation
1 / 5
Title:
Visible Spectrum
Description:
Electromagnetic Spectrum Particle Description of Light Max Planck Albert Einstein Visible Spectrum Electromagnetic Spectrum Particle Description of Light Max Planck ... – PowerPoint PPT presentation
Number of Views:204
Avg rating:3.0/5.0
Title: Visible Spectrum
1
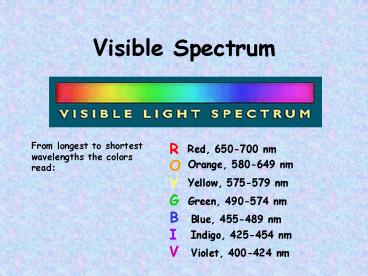
Visible Spectrum
From longest to shortest wavelengths the colors
read
ROYGBIV
Red, 650-700 nm
Orange, 580-649 nm
Yellow, 575-579 nm
Green, 490-574 nm
Blue, 455-489 nm
Indigo, 425-454 nm
Violet, 400-424 nm
2
Electromagnetic Spectrum
Shortest wavelength Greatest frequency
Longest wavelength Lowest frequency
All wavelengths of light travel at the same
speed, the speed of light.
Wavelength and frequency are inversely
proportional.
3
Particle Description of Light
(when the light given off is a stream of
particles - electrons - instead of light waves)
Photoelectric Effect
- emission of electrons from a metal when light
shines on the metal
Example much like a coke machine that requires
an exact coin before it will give back a coke.
Exact energy must be put in for electrons to be
given off.
4
Max Planck
- German scientist
- studied light given off by hot objects
- didnt think waves were continuous
- thought energy was given off in packets he called
quantas - Quantum minimum amount of energy that can be
lost or gained by an atom
Relationship E h?
- E energy in joules
- frequency
- H Plancks constant
5
Albert Einstein
- expanded on Plancks theory
- introduced idea that electromagnetic radiation
has a dual wave-particle nature - each particle carries a quantum of energy
- light acts like waves, but can also be like a
stream of particles - Photon a particle of electromagnetic radiation
having zero mass and carrying a quantum of energy
E1 photon (h) (?)
-where h Plancks constant, ? frequency and E
is the energy of 1 photon in joules