Protons are positive ( ) charged particles found in the nucleus of an atom. - PowerPoint PPT Presentation
Title:
Protons are positive ( ) charged particles found in the nucleus of an atom.
Description:
The Neutron The neutron is a subatomic particle found in the nucleus. 1 neutron = no charge The neutron was discovered in 1932 by James Chadwick. – PowerPoint PPT presentation
Number of Views:180
Avg rating:3.0/5.0
Title: Protons are positive ( ) charged particles found in the nucleus of an atom.
1
Chapter 4 - Part 2
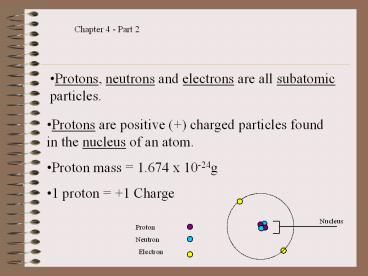
- Protons, neutrons and electrons are all subatomic
particles.
- Protons are positive () charged particles found
in the nucleus of an atom. - Proton mass 1.674 x 10-24g
- 1 proton 1 Charge
Nucleus
Proton
Neutron
Electron
2
- The Neutron
- The neutron is a subatomic particle found in the
nucleus. - 1 neutron no charge
- The neutron was discovered in 1932 by James
Chadwick. - Its mass is almost the same as a proton.
3
- The Electron
- The electron is a subatomic particle found
outside of the nucleus of an atom and it has a
negative (-) charge. - Electrons mass 9.11 x 10-28g
- 1 electron -1 charge
4
Charge
- A proton has a positive charge 1
- An electron has a negative charge -1
- A neutron is neutral. It wont affect overall
charge.
- One positive proton will cancel out one negative
electron leaving an overall charge of Zero
Overall Charge 0
5
Complete the table below
Atom ChargeProtons Charge Neutrons Charge Electrons Overall Charge
5 protons6 neutrons5 electrons
16 protons16 neutrons16 electrons
19 protons20 neutrons19 electrons
6
The Periodic Table
Elements are organized into rows and columns
based on their atomic number.
7
Elements, symbols and masses
8
- Atomic Number
- Atomic number of protons for an element.
- Atoms of different elements has different numbers
of protons. - Each positive charged proton must be balanced by
a negative charged electron. So the atomic
lets you know the of protons and the of
electrons. - Example Sulfur (S) is atomic number 16 because
it has 16 protons. Since it has 16 protons it
has to have 16 electrons.
9
- Mass Number
- The mass number lets you figure out how many
neutrons are present in an element. - Mass of protons of neutrons or mpn
- Neutrons mass - atomic since the atomic
number is the same as the number of protons then
nm-p
10
Mass number continued
- Example Aluminum (Al) has 13 protons.
- Its mass number is 27.
- So, 27-1314 and 14 is the number of neutrons
found in an atom of Aluminum.
11
Atomic Mass Units
- An atomic mass unit (amu) is defined as 1/12th
of a Carbon-12 atom.
- AMUs is the unit of mass used on the periodic
table when looking at an elements atomic mass
number. - That's about the mass of one proton or neutron.
Protons and Neutrons 1 amu each
12
AMUs continued
- 1 atomic mass unit 1.66053886 10-24 kg
- One gram is about 600,000,000,000,000,000,000,000
amu. - Ex Fluorines (F) atomic mass is 19 amu, but
its mass in grams is 3.155 x 10 -28 grams. - Wow--I can see why you'd want to use amu's
instead of grams.
13
- What is the atomic mass of an atom with
- 4 protons and 5 neutrons
- 24 protons and 28 neutrons
- 7 protons and 7 neutrons
- 91 protons and 140 neutrons
14
- Complete the table below
Protons Neutrons Atomic Mass
35 65
10 20
39 70
39 89
15
- Isotopes
- Isotopes are atoms of the same element but have a
different number of neutrons and mass number.
- Example Oxygen (O) has 8 protons but some atoms
of oxygen have a mass of 16, 17 or 18. - So this means the number of neutrons varies
between each isotope. So Oxygen-16 has 8
neutrons, Oxygen-17 has 9 neutrons and Oxygen-18
has 10 neutrons.
16
- Common Isotopes
- The element Carbon (C) can be found as C12, C13
and C14. - How many protons, neutrons and electrons does
each carbon isotope above have? - Answer C12 6,6,6
- C13 6,7,6
- C14 6,8,6
17
Hydrogen Isotopes
- Hydrogen-1 doesnt have any neutrons.
- Hydrogen-2 has 1 neutron
- Hydrogen-3 has 2 neutrons