Introduction to Thermochemistry Chapter 16. 1, pp. 531-534 - PowerPoint PPT Presentation
Title:
Introduction to Thermochemistry Chapter 16. 1, pp. 531-534
Description:
Introduction to Thermochemistry Chapter 16. 1, pp. 531-534 Temperature is a measure of the average kinetic energy of the particles in a sample of matter. – PowerPoint PPT presentation
Number of Views:104
Avg rating:3.0/5.0
Title: Introduction to Thermochemistry Chapter 16. 1, pp. 531-534
1
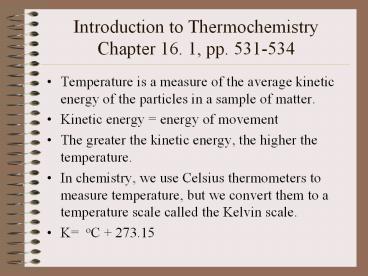
Introduction to ThermochemistryChapter 16. 1,
pp. 531-534
- Temperature is a measure of the average kinetic
energy of the particles in a sample of matter. - Kinetic energy energy of movement
- The greater the kinetic energy, the higher the
temperature. - In chemistry, we use Celsius thermometers to
measure temperature, but we convert them to a
temperature scale called the Kelvin scale. - K oC 273.15
2
Example
- The Celsius temperature is 25 degrees. What is
the Kelvin temperature? - K oC 273.15
- K 25o 273.15
- K 298.15 or 298
- Notice 2 things 1) there is NO unit or degree
marking for Kelvin temperatures 2) We can
usually round the .15 down
3
Measuring Energy Change
- All chemical and physical changes are accompanied
by energy changes. - We usually measure this in an object called a
calorimeter. There are several different types
of calorimeters, but they are all insulated and
enclosed to capture all energy associated with
energy changes.
4
Heat
- Heatthe energy transferred between samples of
matter because of a difference in their
temperatures. - Heat always is spontaneously transferred from the
matter with the highest temperature to the matter
with the lowest temperature. - How much heat is transferred depends upon a lot
of variables.
5
Specific Heat
- Some variables involved in heat transfer include
- 1. Mass of the matter
- 2. Composition of the matter
- 3. Differences in the temperatures of the matter
involved. - Differences in the ability of matter to absorb
heat is due to their composition. We use a
property called specific heat to measure the
difference.
6
Specific Heat
- Specific heat of a substancethe amount of energy
required to raise 1 gram of the substance 1
Celsius degree or 1 Kelvin - Every substance has a different specific heat, so
we can use this property for identification
purposes. - Page 533 in your book has a chart of some
specific heat values.
7
Specific Heat
- Specific heat has units of J/g-K, which is Joules
per gram-Kelvin or we can use J/g-oC (Joules per
gram-Celsius degree) - There are 2 equations we use with specific heat
(Copy these from the board power point does not
show them well)
8
Specific Heat
- cp specific heat
- q energy lost or gained
- M mass of sample
- T change in temperature
- See pages 533-534 for examples of specific heat
problems.