Chemical Equation - PowerPoint PPT Presentation
1 / 15
Title:
Chemical Equation
Description:
Chemical Equation & Reaction Notes Chemical reactions occur when chemical bonds (ionic, covalent) between atoms are broken and new bonds are formed – PowerPoint PPT presentation
Number of Views:36
Avg rating:3.0/5.0
Title: Chemical Equation
1
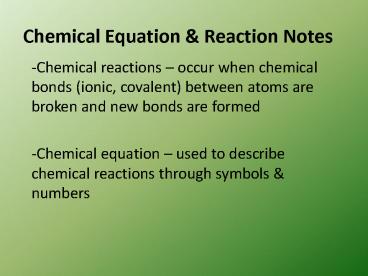
Chemical Equation Reaction Notes
- Chemical reactions occur when chemical bonds
(ionic, covalent) between atoms are broken and
new bonds are formed - Chemical equation used to describe chemical
reactions through symbols numbers
2
(No Transcript)
3
Example of chemical equationaluminum sulfate
plus barium chloride yields aluminum chloride
plus barium sulfate
- Al2(SO4)3 3 BaCl2 ? 2 AlCl3 3
BaSO4 - Label the following
- Reactants - Products -Coefficients
- - Subscripts - Produces or yields
4
Terms or symbols used in equations
- or and - used as divider between
reactants or products - ? yields, reacts, produces, forms,
- reversible reaction
- CATALYST is something that speeds up the
reaction, but it is not used as a chemical part
of the reaction written on top of the yield sign - Heat or ? or MnO2 or
elec
5
More terms and symbols
- (s) solid
- (l) liquid
- (aq) aqueous, dissolved in water
- (g) gas
- NR no reaction
6
(No Transcript)
7
- There are 5 types of chemical reactions
8
1 - Synthesis
- Reactants combine to form 1 compound
- (aka, combination rxn)
- reactant reactant ?
product - Basically A B ? AB
- Ex 2 H2 O2 ? 2 H2O
- Ex C O2 ? CO2
9
Synthesis Reaction (continued)
- Here is another example of a synthesis rxn
10
2 - Decomposition
- occur when a compound breaks up into 2 or more
products (can be elements or compounds) - 1 Reactant ? Product Product
- In general AB ? A B
- Ex 2 H2O ? 2 H2
O2 - Ex 2 HgO ? 2 Hg
O2
11
Decomposition (continued)
- Another view of a decomposition reaction
12
3 - Single Replacement
- occur when one element replaces another in a
compound (aka, single displacement) - AB C ? CB
A - Metals must be more reactive in order to replace
another metal (called the Activity Series) - Zn(s) HCl(aq) ? ZnCl2 H2(g)
13
(No Transcript)
14
4 Double Replacement
- Occurs when 2 ionic compounds switch partners
and produce 2 different compounds - Compound compound ? compound compound
- AB CD ? AD CB
- Ex AgNO3(aq) NaCl(s) ? AgCl(s)
NaNO3(aq)
15
5 - Combustion
- occur when a hydrocarbon reacts with oxygen
- This is also called burning
- In general CxHy O2 ?
CO2 H2O - Products in combustion are ALWAYS carbon dioxide
and water. (incomplete combustion will also
produce carbon monoxide)