Atoms, Elements, Compounds, and Ions - PowerPoint PPT Presentation
1 / 33
Title:
Atoms, Elements, Compounds, and Ions
Description:
Title: PowerPoint Presentation Author: student Last modified by: Chaco, John (Platt) Created Date: 8/27/2002 6:27:18 PM Document presentation format – PowerPoint PPT presentation
Number of Views:70
Avg rating:3.0/5.0
Title: Atoms, Elements, Compounds, and Ions
1
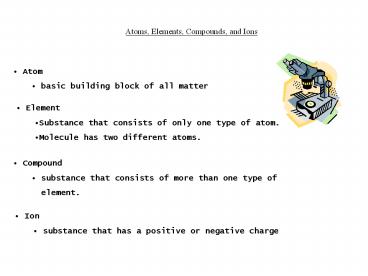
Atoms, Elements, Compounds, and Ions
- Atom
- basic building block of all matter
- Element
- Substance that consists of only one type of atom.
- Molecule has two different atoms.
- Compound
- substance that consists of more than one type of
- element.
- Ion
- substance that has a positive or negative charge
2
Atoms
Atoms have
- A nucleus
- small, heavy part of the atom
- An electron cloud
- large, lightweight part of the atom
3
Nucleus of an Atom
- Nucleus contains
- Protons
- Have a positive charge
- All atoms are distinguished by
- the number of protons it
- has (atomic number)
N
- Neutrons
- Have no charge
- Have same mass as protons
4
Electron Cloud of an Atom
- An electron cloud contains
- Electrons
- have a negative charge
- are contained within shells of the electron cloud
- orbit the nucleus of the atom
- have a very small mass compared to protons and
neutrons
- determine bonding properties of substance
5
Atom Structure
-1
1
1
-1
6
Elements
Elements contain one or more of the same type of
atom!
Examples include
Oxygen 8 protons per atom
Copper 29 protons per atom
Gold 79 protons per atom
7
Compounds
Compounds contain more than one type of atom!
Example of organic compound (a compound with
carbon atoms)
Example of inorganic compound (a compound with no
carbon atoms)
8
-
IONS
An ion is an atom or group of atoms with a
positive or negative charge!!
- A particle with a neutral charge has the same
number of - protons and electrons.
- An ion does not have the same number of
electrons and protons.
- Examples of ions
- He - A helium atom that is missing one
electron. - The atom has one more proton than
electron, - and must have a positive charge.
- CO32- - Carbonate has two more electrons than
protons
9
Andy the Hydrogen Atom
Lets take a look deep inside
10
Andy the Hydrogen Atom
Lets take a look deep inside
11
Andy the Hydrogen Atom
Lets take a look deep inside
12
Andy the Hydrogen Atom
Lets take a look deep inside
13
(No Transcript)
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
This is a Proton. Positive Charge weighs
ONE Tells what type of atom it is GIRL
18
This is a Proton. Positive Charge weighs
ONE Tells what type of atom it is GIRL
19
This is a Proton. Positive Charge weighs
ONE Tells what type of atom it is GIRL
20
This is a Proton. Positive Charge weighs
ONE Tells what type of atom it is GIRL
This is a Neutron No Charge, Weighs One NUN
(does not date)
21
This is an Electron No Weight Negative charge BOY
This is a Proton. Positive Charge weighs
ONE Tells what type of atom it is GIRL
This is a Neutron No Charge, Weighs ONE NUN
(does not date)
22
Now.... Listen to Mr. Chacos ION dating game!
23
Element Guide
24
Element Guide
2
25
Element Guide
2
26
Element Guide
Proton Number-tells what element it is. (also the
atomic number)
2
27
Element Guide
Proton Number-tells what element it is. (also the
atomic number)
2
He
28
Element Guide
Proton Number-tells what element it is. (also the
atomic number)
2
He
29
Element Guide
Proton Number-tells what element it is. (also the
atomic number)
2
He
Explains name of the element.
30
Element Guide
Proton Number-tells what element it is. (also the
atomic number)
2
He
Explains name of the element.
4
31
Element Guide
Proton Number-tells what element it is. (also the
atomic number)
2
He
Explains name of the element.
4
32
Element Guide
Proton Number-tells what element it is. (also the
atomic number)
2
He
Explains name of the element.
4
Explains the total atomic weight-The number of
protons PLUS the number of neutrons.
33
Element Guide
Proton Number-tells what element it is. (also the
atomic number)
2
He
Explains name of the element.
4
Explains the total atomic weight-The number of
protons PLUS the number of neutrons.