Chemical Formulas and Equations - PowerPoint PPT Presentation
1 / 15
Title:
Chemical Formulas and Equations
Description:
Chemical Formulas and Equations ... Other uses: they contain proteases found in meat tenderizers, and contact lens Homework: Complete section assessment 1, ... – PowerPoint PPT presentation
Number of Views:84
Avg rating:3.0/5.0
Title: Chemical Formulas and Equations
1
Chemical Formulas and Equations
2
Physical or Chemical Changes Matter can undergo
two kinds of changes
- Physical changes in a substance only affect
physical properties such as size, shape or
whether a solid, liquid or a gas
- Chemical changes
- Produce new substances that have properties
different form those of the original substances
3
Chemical Changes
- The rust on a bikes handlebars, for example, has
properties different from those of the metal
around it. - A process that produces chemical change is a
Chemical Reaction. - Folding a newspaper in half- C or P
- Lighting a fire- C or P
4
Chemical Equations
- To describe a chemical reaction you must know
which substances react and which are produced - The substances that react are called REACTANTS
- The substances that are produced are called
PRODUCTS - Baking Soda Vinegar Chemical or Physical
5
Chemical Rxn cont.
- The bubbles that form produces a gas and
something else. What goes on in a chemical rxn is
more than what you can see with your eyes. - Chemists are interested in which reactants are
used and the products that are produced. - To do this, a shorthand way of indicating what
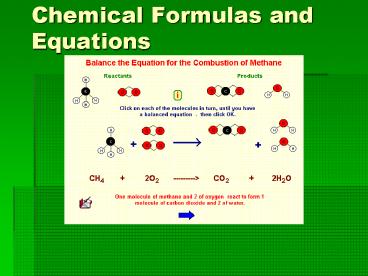
happens is called a chemical equation. - C12H22O11 H2O
CO2
6
Chemical Equations
- The Law of Conservation of Matter states that
nothing is created or destroyed. Therefore, the
mass of the chemical reactants must be the same
mass as the products - Ex Nacl -------- NaCl2 is are the
elements ? - When you write the chemical equation you must
observe the law of conservation of matter,
sometimes this easy - Count the of atoms of each type in the
reactants and the products
7
Balancing Chemical Equations
- Ag H2S -------- Ag2 S H2
- 2 Ag H2S ------- Ag2 H2
- Any Questions? What do you think?
- Complete the following exercise
8
Energy in Chemicals
- Often, energy is released or absorbed during a
chemical rxn. The energy for the welding torch is
released when H and O combine to form water 2H2
O2 ---- 2H2O energy. - This energy comes from chemical bonds that break
to form when atoms gain, lose or share electrons
in the reactants and new bonds form in the
product
9
Energy in chemical rxn
- In rxns that release energy, the products are
more stable and their bonds have less energy than
those of the reactants. The extra energy is
released in other forms such as light, sound and
heat - When energy is absorbed, the reactants are more
stable and their bonds have less energy than
those of the products - 2H2 O2 energy--- 2H2 O2.
10
Energy of Rxns
- Endothermic rxns occur when there is an
absorption of heat energy - Ex Heating a home, cold pack (AlNO3 H20)
- Exothermic rxns occur when there is a release of
heat energy - Ex Burning materials, skid marks from an
accident
11
Activation Energy.
- Before a rxn can start, molecules of the reactant
must bump into each other or collide - This means that there must be a strong collision
that will cause the molecules to speed up - The amount of energy needed to speed up a
chemical rxn is called the Activation Energy - Ex Burning of gasoline.. Thats why signs are
posted at gas stations with warnings!!
12
Reaction Rate
- The rate of Rxn tells how fast a rxn occurs. To
find the rate of a rxn, you can measure either
how quickly one of the reactants is disappearing
or how quickly one of the products is appearing - Factors that affect Reaction rate are
- Temperature changes, Raising Temp, Concentration
of molecules and particle size
13
Energy in Chemicals
- Factors that slow down chemical rxns
- Substances that slow down rxn are called
inhibitors. - Inhibitors do not completely stop a rxn, they
simply slow down its progression of the product. - Substances that speed up a rxn are called
catalyst. It is used to produce the same amount
of product faster
14
Enzymes
- Enzymes are substances that can speed a rxn or
slow down a rxn. - Enzymes make it possible for your body to
function. All enzymes are proteins, but not all
proteins are enzymes. - An enzyme exists to carry out each type or rxn in
your body. - Other uses they contain proteases found in meat
tenderizers, and contact lens
15
Homework
- Complete section assessment 1, pg46 (1-6).
- Complete Physical/chemical Changes activity
handout in 6.1 pH packet - All packets are due 9/23/2010