Bio NOTES: Intro to the Chemistry - PowerPoint PPT Presentation
1 / 14
Title:
Bio NOTES: Intro to the Chemistry
Description:
Bio NOTES: Intro to the Chemistry Your life DEPENDS on chemistry! When you inhale oxygen, your body uses it in chemical reactions! When you eat food, your body breaks ... – PowerPoint PPT presentation
Number of Views:244
Avg rating:3.0/5.0
Title: Bio NOTES: Intro to the Chemistry
1
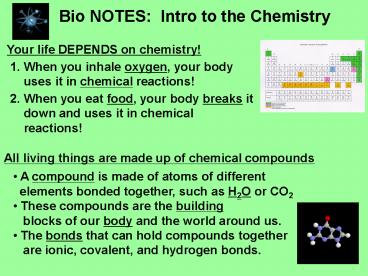
Bio NOTES Intro to the Chemistry
Your life DEPENDS on chemistry!
- When you inhale oxygen, your body uses it in
chemical reactions!
- When you eat food, your body breaks it down and
uses it in chemical reactions!
All living things are made up of chemical
compounds
- A compound is made of atoms of different
- elements bonded together, such as H2O or CO2
- These compounds are the building
- blocks of our body and the world around us.
- The bonds that can hold compounds together
- are ionic, covalent, and hydrogen bonds.
2
3 Types of Bonds
1. Ionic bond forms between oppositely
charged ions
- Ion atom that has lost or
- gained electrons
2. Covalent bond forms when atoms share a
pair of electrons
3. Hydrogen bond forms between slightly
positive hydrogen atoms and slightly
negative atoms
3
WATER
- Water is a polar molecule
- polar has slightly charged regions
- nonpolar does not have charged regions
- Oxygennegative charge
- HydrogenPositive charge
- 2. Water LOVES itself, therefore it clings to
itself! - -This is called Cohesion
- - this creates Surface Tension
- Water also loves other substances!
- -This is called adhesion
- -This causes capillary action
4
3. Water is held together by hydrogen bonds
Negative Oxygen end of one water molecule is
attracted to the Positive Hydrogen end of another
water molecule to form a HYDROGEN BOND
5
WATER
4. Many compounds dissolve in water. Water is a
universal Solvent
- A solution is formed when one
- substance dissolves in another.
- - A solvent dissolves other substances.
- A solute dissolves in a solvent
water loving
hydrophilic hydrophobic
water fearing
6
Water is Less Dense as a Solid
- Ice is less dense as a solid than as a liquid
(ice floats) - Liquid water has hydrogen bonds that are
constantly being broken and reformed. - Frozen water forms a crystal-like lattice whereby
molecules are set at fixed distances.
7
- Water is Less Dense as a Solid
- Which is ice and which is water?
8
- Water is Less Dense as a Solid
Water
Ice
9
- Properties of Water Flapbook
- Fold a sheet of paper hot dog or landscape
style. - Divide the sheet of paper into four equal
sections. - Label each section as follows Solvent, cohesion,
polar molecule, density - Then cut each segment (top only to form flaps).
- Under each flap include the following information
for each unusual property of water - Meaning of the term
- Examples of why this property of water is
important to life - Picture of this property in nature
10
pH
- Reminder Water molecules are held together by
___________________ bonds. - These are relatively __________ bonds.
- These bonds are constantly _________ and
_____________. - When water breaks apart, there is a way to
represent this using chemical equations.
(Dissociation of water)
11
Acids, Bases and pH
- water molecules dissociates into a Hydrogen Ion
(H) and a Hydroxide Ion (OH-) - Hydrogen Ion
Hydroxide Ion - Acid Base
H2O ? H OH-
12
The pH Scale
Some compounds form acids or bases
- An acid releases a hydrogen ion when it
dissolves in water. - - high H concentration
- - pH less than 7
- A base removes hydrogen ions from a solution
- low H concentration - pH greater than 7
- A neutral solution has a pH of 7
13
A B
Left to right
14
(No Transcript)