Feldspar Group - PowerPoint PPT Presentation
Title:
Feldspar Group
Description:
... (OH) See handout for more information General formula: W0 ... and gibbsite-type dioctahedral a1 a2 Phyllosilicates Muscovite: K Al2 [Si3AlO10] (OH)2 ... – PowerPoint PPT presentation
Number of Views:233
Avg rating:3.0/5.0
Title: Feldspar Group
1
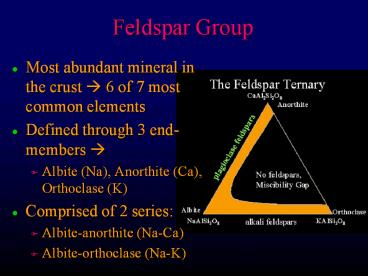
Feldspar Group
- Most abundant mineral in the crust ? 6 of 7 most
common elements - Defined through 3 end-members ?
- Albite (Na), Anorthite (Ca), Orthoclase (K)
- Comprised of 2 series
- Albite-anorthite (Na-Ca)
- Albite-orthoclase (Na-K)
2
Tectosilicates
- Feldspars
Substitute Al3 for Si4 allows Na or K to be
added Albite-Orthoclase
Substitute two Al3 for Si4 allows Ca2 to be
added Albite-Anorthite
Albite NaAlSi3O8
3
Feldspar Group Albite-Anorthite series
- Complete solid solution ? Plagioclase Feldspars
- 6 minerals
- Albite (Na)
- Oligoclase
- Andesine
- Labradorite
- Bytownite
- Anorthite (Ca)
- Albite-Anorthite double duty
- End-members (Pure Na or Ca)
- Minerals 90-99.99 Na or Ca
- Notation
- AnxAby ? An20Ab80Oligoclase
4
Feldspar Group Albite-Anorthite series
- Optical techniques to distinguish between
plagioclase feldspars - Michel-Levy Method uses extinction angles of
twinned forms to determine An-Ab content - Combined Carlsbad-Albite Method ? uses
Michel-Levy technique for both sides of a twin
form
5
Feldspar Group Albite-Orthoclase series
- Several minerals Alkali Feldspars
- High T minerals
- Sanidine
- Anorthoclase
- Monalbite
- High Albite
- Low Temperature exsolution at solvus
- Chicken soup separation
- Forms 2 minerals, in igneous rocks these are
typically intergrowths, or exsolution lamellae
perthitic texture
6
Alkali Feldspar Exsolution
- Melt cools past solvus (line defining miscibility
gap) - Anorthoclase, that had formed (through
liquidus/solidus) separates (if cooling is slow
enough) to form orthoclase and low albite - In hand sample schiller effect ? play of colors
caused by lamellae
7
Alkali Feldspar lamellae
8
Feldspathoid Group
- Very similar to feldspars and zeolites
- Include Nepheline, Analcime, and Leucite
- Also framework silicates, but with another Al
substitution for Si - Only occur in undersaturated rocks (no free
Quartz, Si-poor) because they react with SiO2 to
form feldspars
9
- Nesosilicates independent SiO4 tetrahedra
b
M1 in rows and share edges M2 form layers in a-c
that share corners Some M2 and M1 share edges
a
Olivine (001) view blue M1 yellow M2
10
- Olivine complete solid solution
- Forsterite-Fayalite ? FoxFay
- Fayalite Fe end-member
- Forsterite Mg end-member
- Olivine Occurrences
- Principally in mafic and ultramafic igneous and
meta-igneous rocks - Fayalite in meta-ironstones and in some alkalic
granitoids - Forsterite in some siliceous dolomitic marbles
- Monticellite CaMgSiO4
- Ca ? M2 (larger ion, larger site)
- High grade metamorphic siliceous carbonates
11
Distinguishing Forsterite-Fayalite
- Petrographic Microscope
- Index of refraction ? careful of zoning!!
- 2V different in different composition ranges
- Pleochroism/ color slightly different
- Spectroscopic techniques many ways to determine
Fe vs. Mg - Same space group (Pbnm), Orthorhombic, slight
differences in unit cell dimensions only
12
- Inosilicates single chains- pyroxenes
b
Diopside CaMg Si2O6
a sin?
Where are the Si-O-Si-O chains??
Diopside (001) view blue Si purple M1 (Mg)
yellow M2 (Ca)
13
- Inosilicates single chains- pyroxenes
b
a sin?
Diopside (001) view blue Si purple M1 (Mg)
yellow M2 (Ca)
14
- Inosilicates single chains- pyroxenes
b
a sin?
Diopside (001) view blue Si purple M1 (Mg)
yellow M2 (Ca)
15
- Inosilicates single chains- pyroxenes
b
a sin?
Diopside (001) view blue Si purple M1 (Mg)
yellow M2 (Ca)
16
- Inosilicates single chains- pyroxenes
b
a sin?
Diopside (001) view blue Si purple M1 (Mg)
yellow M2 (Ca)
17
- Inosilicates single chains- pyroxenes
b
a sin?
Diopside (001) view blue Si purple M1 (Mg)
yellow M2 (Ca)
18
- Inosilicates single chains- pyroxenes
Perspective view
Diopside (001) view blue Si purple M1 (Mg)
yellow M2 (Ca)
19
- Inosilicates single chains- pyroxenes
SiO4 as polygons (and larger area)
IV slab
VI slab
IV slab
a sin?
VI slab
IV slab
VI slab
IV slab
b
Diopside (001) view blue Si purple M1 (Mg)
yellow M2 (Ca)
20
- Inosilicates single chains- pyroxenes
M1 octahedron
21
- Inosilicates single chains- pyroxenes
M1 octahedron
22
- Inosilicates single chains- pyroxenes
()
M1 octahedron
() type by convention
23
- Inosilicates single chains- pyroxenes
M1 octahedron This is a (-) type
(-)
24
- Inosilicates single chains- pyroxenes
T M1 T Creates an I-beam like unit in the
structure.
25
- Inosilicates single chains- pyroxenes
T M1 T Creates an I-beam like unit in the
structure
26
Inosilicates single chains- pyroxenes
The pyroxene structure is then composed of
alternating I-beams Clinopyroxenes have all
I-beams oriented the same all are () in this
orientation
Note that M1 sites are smaller than M2 sites,
since they are at the apices of the tetrahedral
chains
27
Inosilicates single chains- pyroxenes
The pyroxene structure is then composed of
alternation I-beams Clinopyroxenes have all
I-beams oriented the same all are () in this
orientation Orthopyroxenes have alternating ()
and (-) orientations
28
Inosilicates single chains- pyroxenes
Tetrehedra and M1 octahedra share tetrahedral
apical oxygen atoms
29
Inosilicates single chains- pyroxenes
The tetrahedral chain above the M1s is thus
offset from that below The M2 slabs have a
similar effect The result is a monoclinic unit
cell, hence clinopyroxenes
() M2
c
a
() M1
() M2
30
Inosilicates single chains- pyroxenes
Orthopyroxenes have alternating () and (-)
I-beams the offsets thus compensate and result
in an orthorhombic unit cell
c
(-) M1
() M2
a
() M1
(-) M2
31
Pyroxene Chemistry
- The general pyroxene formula
- W1-P (X,Y)1P Z2O6
- Where
- W Ca Na
- X Mg Fe2 Mn Ni Li
- Y Al Fe3 Cr Ti
- Z Si Al
- Anhydrous so high-temperature or dry
conditions favor pyroxenes over amphiboles
32
Pyroxene Chemistry
- The pyroxene quadrilateral and opx-cpx solvus
- Coexisting opx cpx in many rocks (pigeonite
only in volcanics)
Wollastonite Ca2Si2O6
- Orthopyroxenes solid soln between
Enstatite-Ferrosilite - Clinopyroxenes solid soln between
Diopside-Hedenbergite
Hedenbergite CaFeSi2O6
Diopside CaMgSi2O6
clinopyroxenes
Joins lines between end members limited
mixing away from join
pigeonite
orthopyroxenes
Ferrosilite Fe2Si2O6
Enstatite Mg2Si2O6
33
Orthopyroxene - Clinopyroxene
- OPX and CPX have different crystal structures
results in a complex solvus between them - Coexisting opx cpx in many rocks (pigeonite
only in volcanics)
pigeonite
1200oC
orthopyroxenes
clinopyroxenes
1000oC
CPX
Solvus
800oC
(Mg,Fe)2Si2O6
Ca(Mg,Fe)Si2O6
OPX
OPX
CPX
34
Orthopyroxene Clinopyroxenesolvus T dependence
- Complex solvus the stability of a particular
mineral changes with T. A different minerals
stability may change with T differently - OPX-CPX exsolution lamellae ? Geothermometer
CPX
CPX
Hd
Di
Di
Hd
augite
augite
Subcalcic augite
Miscibility Gap
Miscibility Gap
pigeonite
pigeonite
orthopyroxene
orthopyroxene
Fs
En
Fs
En
OPX
OPX
800ºC
1200ºC
Pigeonite orthopyroxene
35
Pyroxenoids
Ideal pyroxene chains with 5.2 A repeat (2
tetrahedra) become distorted as other cations
occupy VI sites
Wollastonite (Ca ? M1) ? 3-tet repeat
Rhodonite MnSiO3 ? 5-tet repeat
Pyroxmangite (Mn, Fe)SiO3 ? 7-tet repeat
Pyroxene 2-tet repeat
36
- Inosilicates double chains- amphiboles
b
Tremolite Ca2Mg5 Si8O22 (OH)2
a sin?
Tremolite (001) view blue Si purple M1
rose M2 gray M3 (all Mg) yellow M4 (Ca)
37
- Inosilicates double chains- amphiboles
b
Hornblende (Ca, Na)2-3 (Mg, Fe, Al)5
(Si,Al)8O22 (OH)2
a sin?
Hornblende (001) view dark blue Si, Al
purple M1 rose M2 light blue M3 (all
Mg, Fe) yellow ball M4 (Ca) purple ball
A (Na) little turquoise ball H
38
- Inosilicates double chains- amphiboles
Hornblende (Ca, Na)2-3 (Mg, Fe, Al)5
(Si,Al)8O22 (OH)2
Same I-beam architecture, but the I-beams are
fatter (double chains)
Hornblende (001) view dark blue Si, Al
purple M1 rose M2 light blue M3 (all
Mg, Fe)
39
- Inosilicates double chains- amphiboles
b
Hornblende (Ca, Na)2-3 (Mg, Fe, Al)5
(Si,Al)8O22 (OH)2
Same I-beam architecture, but the I-beams are
fatter (double chains)
a sin?
All are () on clinoamphiboles and alternate in
orthoamphiboles
Hornblende (001) view dark blue Si, Al
purple M1 rose M2 light blue M3 (all
Mg, Fe) yellow ball M4 (Ca) purple ball
A (Na) little turquoise ball H
40
- Inosilicates double chains- amphiboles
Hornblende (Ca, Na)2-3 (Mg, Fe, Al)5
(Si,Al)8O22 (OH)2 M1-M3 are small sites M4 is
larger (Ca) A-site is really big Variety of
sites ? great chemical range
Hornblende (001) view dark blue Si, Al
purple M1 rose M2 light blue M3 (all
Mg, Fe) yellow ball M4 (Ca) purple ball
A (Na) little turquoise ball H
41
- Inosilicates double chains- amphiboles
Hornblende (Ca, Na)2-3 (Mg, Fe, Al)5
(Si,Al)8O22 (OH)2 (OH) is in center of
tetrahedral ring where O is a part of M1 and M3
octahedra
(OH)
Hornblende (001) view dark blue Si, Al
purple M1 rose M2 light blue M3 (all
Mg, Fe) yellow ball M4 (Ca) purple ball
A (Na) little turquoise ball H
42
Amphibole Chemistry
See handout for more information General
formula W0-1 X2 Y5 Z8O22 (OH, F, Cl)2 W
Na K X Ca Na Mg Fe2 (Mn Li) Y Mg
Fe2 Mn Al Fe3 Ti Z Si Al Again, the
great variety of sites and sizes ? a great
chemical range, and hence a broad stability
range The hydrous nature implies an upper
temperature stability limit
43
Amphibole Chemistry
Ca-Mg-Fe Amphibole quadrilateral (good analogy
with pyroxenes)
Tremolite
Ferroactinolite
Actinolite
Ca2Mg5Si8O22(OH)2
Ca2Fe5Si8O22(OH)2
Clinoamphiboles
Cummingtonite-grunerite
Anthophyllite
Fe7Si8O22(OH)2
Mg7Si8O22(OH)2
Orthoamphiboles
Al and Na tend to stabilize the orthorhombic form
in low-Ca amphiboles, so anthophyllite ? gedrite
orthorhombic series extends to Fe-rich gedrite
in more Na-Al-rich compositions
44
Amphibole Chemistry
Hornblende has Al in the tetrahedral
site Geologists traditionally use the term
hornblende as a catch-all term for practically
any dark amphibole. Now the common use of the
microprobe has petrologists casting hornblende
into end-member compositions and naming
amphiboles after a well-represented
end-member. Sodic amphiboles Glaucophane
Na2 Mg3 Al2 Si8O22 (OH)2 Riebeckite Na2
Fe23 Fe32 Si8O22 (OH)2 Sodic amphiboles are
commonly blue, and often called blue amphiboles
45
Inosilicates
a
-
-
-
-
-
-
Clinopyroxene
Clinoamphibole
a
-
-
-
-
-
-
Orthopyroxene
Orthoamphibole
- Pyroxenes and amphiboles are very similar
- Both have chains of SiO4 tetrahedra
- The chains are connected into stylized I-beams by
M octahedra - High-Ca monoclinic forms have all the T-O-T
offsets in the same direction - Low-Ca orthorhombic forms have alternating ()
and (-) offsets
46
- Inosilicates
pyroxene
amphibole
b
a
Cleavage angles can be interpreted in terms of
weak bonds in M2 sites (around I-beams instead of
through them) Narrow single-chain I-beams ? 90o
cleavages in pyroxenes while wider double-chain
I-beams ? 60-120o cleavages in amphiboles
47
Tectosilicates
After Swamy and Saxena (1994) J. Geophys. Res.,
99, 11,787-11,794.
48
Tectosilicates
- Low Quartz
001 Projection Crystal Class 32
49
Tectosilicates
- High Quartz at 581oC
001 Projection Crystal Class 622
50
Tectosilicates
- Cristobalite
001 Projection Cubic Structure
51
Tectosilicates
- Stishovite
High pressure ? SiVI
52
Tectosilicates
- Low Quartz
Stishovite
SiIV
SiVI
53
Phyllosilicates
SiO4 tetrahedra polymerized into 2-D sheets
Si2O5 Apical Os are unpolymerized and are
bonded to other constituents
54
Phyllosilicates
Tetrahedral layers are bonded to octahedral
layers (OH) pairs are located in center of T
rings where no apical O
55
Phyllosilicates
Octahedral layers can be understood by analogy
with hydroxides
Brucite Mg(OH)2 Layers of octahedral Mg in
coordination with (OH) Large spacing along c due
to weak van der waals bonds
c
56
Phyllosilicates
a2
a1
Gibbsite Al(OH)3 Layers of octahedral Al in
coordination with (OH) Al3 means that only 2/3
of the VI sites may be occupied for
charge-balance reasons Brucite-type layers may
be called trioctahedral and gibbsite-type
dioctahedral
57
(No Transcript)
58
Phyllosilicates
T O T K T O T K T O T
Muscovite K Al2 Si3AlO10 (OH)2 (coupled K -
AlIV) T-layer - diocathedral (Al3) layer -
T-layer - K
K between T - O - T groups is stronger than vdw
59
Phyllosilicates
T O T K T O T K T O T
Phlogopite K Mg3 Si3AlO10 (OH)2 T-layer -
triocathedral (Mg2) layer - T-layer - K
K between T - O - T groups is stronger than vdw
60
Igneous Minerals
- Quartz, Feldspars (plagioclase and alkaline),
Olivines, Pyroxenes, Amphiboles - Accessory Minerals mostly in small quantities
or in special rocks - Magnetite (Fe3O4)
- Ilmenite (FeTiO3)
- Apatite (Ca5(PO4)3(OH,F,Cl)
- Zircon (ZrSiO4)
- Sphene (a.k.a. Titanite) (CaTiSiO5)
- Pyrite (FeS2)
- Fluorite (CaF2)