Midterm%20Review - PowerPoint PPT Presentation
Title:
Midterm%20Review
Description:
As it falls, it changes into kinetic energy because of its motion. ... Mechanical energy Explain the transformation of energy taking place as a car burns up gasoline. – PowerPoint PPT presentation
Number of Views:104
Avg rating:3.0/5.0
Title: Midterm%20Review
1
Physical Science
S8P1. Students will examine the scientific view
of the nature of matter. S8P2. Students will be
familiar with the forms and transformations of
energy. S8P4. Students will explore the wave
nature of sound and electromagnetic radiation.
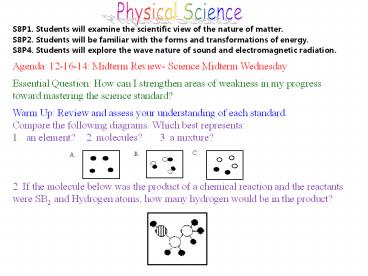
- Agenda 12-16-14 Midterm Review- Science Midterm
Wednesday - Essential Question How can I strengthen areas of
weakness in my progress toward mastering the
science standard? - Warm Up Review and assess your understanding of
each standard. - Compare the following diagrams. Which best
represents - an element? 2. molecules? 3. a mixture?
- 2. If the molecule below was the product of a
chemical reaction and the reactants were SB2 and
Hydrogen atoms, how many hydrogen would be in the
product?
2
Midterm Review
- Check your answer and make corrections or
additions as we review. - Raise your hand if you have a question.
3
- Which tools are best for volume? Density? Mass?
- Volume--beakers, graduated cylinders, flasks.
- Density--graduated cylinders and triple beam
balance. - Mass--triple beam balance.
- 2. Use the table below to answer the question.
- 1981 Pennies 1986 Pennies
- Mass Volume Mass
Volume - 4.5 g .8
3.5 g .8 - In 1982, the composition of U.S. pennies were
changed. According to the chart, how did the
pennies change in 1986? - The 1986 pennies have a lower mass 4.5 vs. 3.5
4
- 3. A spring scale works because the spring
stretches the same amount for each additional
unit of weight that is hung from it. The chart
below shows the length of a particular spring
with different weights hanging from it. What
would the length be if the weight was 600 grams? - Weight 100 g 200g 300g 400g
- Length 6 cm 8 cm 10 cm 12 cm
- 16 cm
- 4. Alison measured the mass of a sample as 3.12
kg. What is the same mass in grams? - 3, 120 grams
- 5. Cara wants to compare the masses of two
different mineral samples. Which would be the
best tool to use? - triple beam balance
5
- 6. What is the main purpose of charts, graphs,
and tables? - To show information in a clear and precise way.
To make reading it easier. - 7. What is scientific method? Name 5 of the
steps. - Scientific Method is the organized method
scientists use to investigate problems. - Research, hypothesis, experiment, analyze, and
form a conclusion. - 8. What is a hypothesis?
- A reasonable and educated guess based on what
you know and what you observe. - 9. What is a constant?
- Variables that do not change in an experiment.
6
- 10. What is the difference between an independent
and dependent variable? - Independent variable--variables changed
in the - experiment.
- Dependent variable---variables that are
changed as a - result of the change in the independent
variable. - 11. Name the chemical elements that can be found
in the following chemical formula H2SO4 - How many atoms?
- 7 atoms
- How many molecules?
- 1 molecule.
- Extra Understanding How many elements?
- 3 Hydrogen, Sulfur, and Oxygen
7
12. Name the parts of the atom and list what
charges they have in a picture. Proton
positive Neutron neutral Electron negative
8
13. Define atomic number number of protons in
the nucleus of a n atom atomic mass number of
protons number of neutrons in the nucleus of an
atom element symbol abbreviation example
Nitrogen-N or Iron-Fe Draw an example of an
element box. atomic number atomic mass
element symbol
9
- 14. What do groups represent on the periodic
table of elements? Vertical column and have
similar chemical properties. - 15. What do periods represent on the periodic
table? - Horizontal rows by energy level, increasing
atomic number. - 16. How do you find the atomic mass?
- Average number of proton neutrons.
10
- 17. List the properties of metals, nonmetals,
metalloids, and inert gases.
Metals Solid at room temperature (except mercury). 2. Malleable and ductile (can be shaped and drawn into wire). 3. Have luster (shinny). Nonmetals Most are gases (except bromine). Brittle Dull Poor conductors Gain electrons in chemical reactions. Metalloids 1. Have properties of both metals and nonmetals. Inert Gases Also known as noble gases. Least reactive
11
- 18. What is a compound?
- A pure substance that forms when two or more
elements join together. - 19. What is the difference between a compound,
solution and a mixture? Compound a substance
produced when elements combine and whose
properties are different from each of the
elements in it. - ex. H2O
- Mixture a combination of compounds and
elements that has not formed a new substance.
Ex. Chex Mix (Heterogeneous) - Solution Homogenous mixture.
12
- 20. What would you classify lemonade as?
- Solution
- 21. What are heterogeneous and homogenous
mixtures? - Heterogeneous unevenly mixed- You can see the
different substances. - Homogenous evenly mixed- You cant see the
different substances. - 22. Define matter.
- Anything that has mass and takes up space.
13
- 23. Define the states of matter and the molecular
structure. Solids molecules packed together
tight - Liquids particles are less densely packed than
a solid Gas weak energy, particles move
freely. - 24. How is a solid changed to a liquid?
- By melting- adding thermal energy
- 25. Define evaporation and condensation.
- Condensation when gas vapors cool and become
liquid. - Evaporation liquid heats up and becomes gas.
14
- 26. What is a physical property of matter?
- Physical property is a characteristic that can
be observed without changing the identity of a
substance. - Name those in your text.
- Ex Mass, color, volume, hardness, and magnetism
- What is a chemical property?
- Chemical property is a characteristic that
describes how a substance will interact during a
chemical reaction. New substances are formed. - Give examples. Ex reactivity ability to burn,
rust, react to light, react with acids.
15
27. What is the pH scale? The pH scale measures
how acidic or basic a substance is. The pH scale
ranges from 0 to 14. A pH of 7 is neutral. A pH
less than 7 is acidic. A pH greater than 7 is
basic.
16
- 28. What is a physical change and chemical
change? - Physical Change alters the physical
properties of a substance without changing the
identity of the substance. - Ex melting ice or evaporation
- Chemical change substance changed into a new
substance with different properties - Ex. sugar, eggs, and flour create a batter.
Baking the cake batter creates a cake. - 29. How would you describe a match being lit and
a nail rusting? Chemical changes - -a match being lit combustion (burning)
- -nail rusting oxidation (rust)- Iron reacts with
oxygen
17
- 30. What is the difference between the Kelvin and
Celsius scale? Degrees Increase/decrease by the
same amount. - Celsius has negative degrees.
- Kelvin is usually used to measures extreme
temperatures like its lowest absolute zero
(theoretical motionless particles). - Explain how a hydroelectric plant (dam) turns
potential energy into electrical energy. - The water has gravitational potential energy due
to its position (height). As it falls, it
changes into kinetic energy because of its
motion. As the water moves the turbines, it is
mechanical energy. A generator transforms this
work into electrical energy. - potential kinetic mechanical
electrical
18
- 32. Hot chocolate and ice water are placed inside
a container. What will eventually happen to the
temperature of the hot chocolate and ice water? - The hot chocolate will lose heat and the ice
water will gain heat until the temperatures are
equal. - 33. A girl is swinging back and forth on a swing
set. She swings forward at her highest point and
is about to jump off. What type of energy does
she have? - She has potential energy at the highest point on
the swing. Remember objects have the greatest
potential energy when they are at the greatest
height. - A humpback whale is leaping out of the water.
Explain what type of energy the whale has as he
leaps out of the water. - Kinetic energy, but as it gains height
potential.
19
- A man lifts a heavy bucket by pulling upward on
the handle. As the man pulls on the handle and
lifts the bucket what kind of energy is being
applied to the bucket? - Mechanical energy
- Explain the transformation of energy taking place
as a car burns up gasoline. - The gasoline burning is chemical energy.
Chemical energy is turned to mechanical energy
as the car moves. Some is as changed to thermal
energy. - Define conduction and convection and give an
example of each. - Conduction is the transfer of heat by
direct contact of particles. - Example Heating a pan on the stove.
- Convection when heat is transferred in fluids
or gases by currents moving in a circular motion.
- Example water heating up on the stove.
20
- Define and give an example of chemical energy,
nuclear energy, mechanical energy, thermal
energy, electromagnetic energy, and electrical
energy. - Chemical energy energy stored in chemical
bonds. - Examples The flame of a candle or digesting
food - Nuclear energy energy stored in the nucleus
of an atom as a result of the nuclear forces. - Examples Nuclear power plant (fission). Stars
burning (fusion) - Mechanical energy the sum of an objects
potential and kinetic energy - Example throwing a ball in the air.
- Thermal energy the total amount of energy of
an object due to the motion of the particles. - Example a cup of hot chocolate.
- Electromagnetic energy travels in waves. It
has some electrical and magnetic properties - Example X-rays, light.
- Electrical energy the energy of moving
charges (electrons). - Example Most of our appliances/electronics use
electrical energy toasters, lights, televisions,
etc.
21
- 39. Describe the Law of Conservation of Energy.
- Energy changes form, but is never created or
destroyed. - 40. Describe electromagnetic waves. What are the
7 major parts of electromagnetic waves? - Electromagnetic waves do not require a medium
in which to travel. - Radio, microwave, infrared, visible light,
ultraviolet, x-ray, and gamma rays. - 41. Describe mechanical waves.
- Requires a medium in which to travel.
- 42. What makes electromagnetic and mechanical
waves different? Mechanical waves use only matter
for travel and electromagnetic use matter and
space for travel.
22
- 43. Draw a transverse wave and diagram the
following parts amplitude, wavelength, crest,
and trough. Define each part of the wave. - Amplitude a measure of how high crests are the
greater the amplitude, the more energy a wave
carries - Wavelengthdistance from the top of one crest to
the top of the next crest or from the bottom of
one trough to the bottom of the next trough - Crest highest part of wave
- Trough lowest part of wave
23
- 44. Draw a longitudinal wave and diagram the
following parts rarefaction compression.