Exp 14B: Determining an Equilibrium Constant - PowerPoint PPT Presentation
Title:
Exp 14B: Determining an Equilibrium Constant
Description:
Exp 14B: Determining an Equilibrium Constant Le Chatelier's Principle In 1884, the French chemist Henri Le Chatelier suggested that equilibrium systems tend to ... – PowerPoint PPT presentation
Number of Views:106
Avg rating:3.0/5.0
Title: Exp 14B: Determining an Equilibrium Constant
1
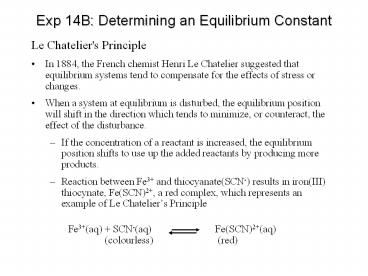
Exp 14B Determining an Equilibrium Constant
- Le Chatelier's Principle
- In 1884, the French chemist Henri Le Chatelier
suggested that equilibrium systems tend to
compensate for the effects of stress or changes. - When a system at equilibrium is disturbed, the
equilibrium position will shift in the direction
which tends to minimize, or counteract, the
effect of the disturbance. - If the concentration of a reactant is increased,
the equilibrium position shifts to use up the
added reactants by producing more products. - Reaction between Fe3 and thiocyanate(SCN-)
results in iron(III) thiocynate, Fe(SCN)2, a red
complex, which represents an example of Le
Chateliers Principle - Fe3(aq) SCN-(aq) Fe(SCN)2(aq)
- (colourless) (red)
2
Determining an Equilibrium Constant Le
Chatelier's Principle
- Changes in ConcentrationConsider the system at
equilibrium - Fe3(aq) SCN-(aq) Fe(SCN)2(aq)
- (colourless) (red)
- Increasing concentration of Fe3(aq) or SCN-(aq)
- results in the equilibrium position moving to the
right - use up some of the additional reactants and
producing more Fe(SCN)2(aq) - solution will become darker red (more Fe(SCN)2).
- Decreasing concentration of Fe3(aq) or SCN-(aq)
- results in the equilibrium position moving to the
left - produces more Fe3(aq) and SCN-(aq).
- the solution will become less red as
Fe(SCN)2(aq) is consumed.
3
Determining an Equilibrium Constant Le
Chatelier's Principle
- Equilibrium constant Keq
- Fe3(aq) SCN-(aq) Fe(SCN)2(aq)
- (colourless) (red)
- Keq Fe(SCN)2eq
- Fe3eq SCN-eq
- How do we measure concentrations?
- Absorption of light
- Applying Beers Law
- absorption of light at a specific wavelength is
proportional to the concentration of a solution
4
Absorption of light by atoms and molecules
Transmission ratio of transmitted
light/incident light I/Io Beers
Law Absorption amount of light absorbed by
solution log Io/I ellc
5
Beers Law
- Transmission I/Io
- Absorption -log T log Io/I
- Beers Law
- A el l c k c
- A absorption of light
- l length of light path
- c concentration
- el molar absorptivity or molar absorption
coefficient - k el l absorption constant
6
Determining an Equilibrium Constant
- Fe3(aq) SCN-(aq) Fe(SCN)2(aq)
- (colourless) (red)
- Experimental
- Measure absorbance of a series of solutions with
different known concentrations of the complex
ion, Fe(SCN)2 - Problem
- Changing concentration of reactants changes
concentration of complex product Fe(SCN)2 is
participant in reaction! - Solution
- Use excess of one of the reactants, so the other
reactant becomes limiting - Use excess SCN-, then Fe3 is limiting reactant
- ? Fe(SCN)2formed Fe3initial
7
AnalysisDetermining absorption constant k
- Measure samples in spectrophotometer at 450 nm
(absorption maximum for Fe(SCN)2) - Plot absorption vs. Fe(SCN)2formed
- Determine absorption constant k slope of curve
- Use A k c, or c A/k
8
AnalysisDetermining Equilibrium Constant K
- Measure A450 nm of samples with different
concentrations of reactants - Calculate Fe(SCN)2, Fe3i, Fe3eq, SCN-i
and SCN-eq - - Fe3i SCN-i 0.0025 M x 1.0 mL/7.0 mL
3.6 x 10-4 M - - Fe(SCN)2 A/k
- - Fe3eq SCN-eq Fe3i - Fe(SCN)2
- 3.6 x 10-4 M A/k X M
- ? Keq Fe(SCN)2eq/Fe3eq SCN-eq
9
Exp 14B Determining an Equilibrium Constant
Part 1 Experimental - Determining k in Beers Law
- Step 1 make a dilution of 0.0025 M Fe(NO3)3 to
0.0001 M 0.0025 M x (4.0 mL/100 mL) - Use a 5-mL Mohr pipet to add 4.0 mL of 0.0025 M
Fe(NO3)3 to a 100-mL volumetric flask - Add 0.1 M HNO3 until exactly 100 mL. Mix
- Rinse the pipet with this solution
- Add the specified amounts from the table below to
5 numbered test tubes
Test Tube No Diluted Fe(NO3)3 (mL) (0.0001 M) 1 M KSCN (ml) 0.1 M HNO3 (mL) Total Volume (mL) Concentration Fe(SCN)2
1 1.0 5.0 4.0 10.0 0.0001 M (1.0 mL/ 10 mL) 1.0 10-5 M
2 2.0 5.0 3.0 10.0
3 3.0 5.0 2.0 10.0
4 4.0 5.0 1.0 10.0
5 5.0 5.0 0 10.0
10
Exp 14B Determining an Equilibrium Constant
Part 1 Analysis - Determining k (absorption
constant)
Test Tube No Fe(SCN)2 Absorption
1 1.0 10-5 M
2
3
4
5
11
Exp 14B Determining an Equilibrium Constant
Part 1 Analysis - Determining k (absorption
constant)
- Plot Fe(SCN)2 vs Absorption
- Fe(SCN)2 on X-axis
- Absorption on Y-axis
- Slope k absorption constant
Line of best fit
Absorption
k slope Abs/Fe(SCN)2
Fe(SCN)2
12
Exp 14B Determining an Equilibrium Constant
Part 2 Experimental - Determining equilibrium
constant Kc
Test Tube No 0.0025 M Fe(NO3)3 (mL) 0.0025 M KSCN (mL) 0.1 M HNO3 (mL) Total Volume (mL)
6 1.0 1.0 5.0 7.0
7 1.0 1.5 4.5 7.0
8 1.0 2.0 4.0 7.0
9 1.0 2.5 3.5 7.0
10 1.0 3.0 3.0 7.0
11 2.0 1.0 4.0 7.0
12 2.0 1.5 3.5 7.0
13 2.0 2.0 3.0 7.0
14 2.0 2.5 2.5 7.0
15 2.0 3.0 2.0 7.0
Total Vol. 5 10 20
13
Exp 14B Determining an Equilibrium Constant
Part 2 Experimental - Determining equilibrium
constant Kc
Test Tube No Absorption
6
7
8
9
10
Test Tube No Absorption
11
12
13
14
15
14
Exp 14B Determining an Equilibrium Constant
Part 2 Analysis - Determining equilibrium
constant Kc
Test Tube Starting Fe3 Starting SCN- Equilibrium Fe(SCN)2 Equilibrium Fe3 Equilibrium SCN- Kc
6
7
8
9
10
11
12
13
14
15
Average
15
Exp 14B Determining an Equilibrium Constant
Part 2 Analysis - Determining equilibrium
constant Kc
- Calculation of concentration
- Tube 6
- starting Fe3 SCN-
- Fe(SCN)2 Absorption/slope Abs/k
- Equilibrium Fe3 Fe3i - Fe(SCN)2e
- Equilibrium SCN- equilibrium Fe3
- Equilibrium constant K Fe(SCN)2e / Fe3e
SCN-e
16
Exp 14B Determining an Equilibrium Constant
Part 2 Analysis - Determining equilibrium
constant Kc
- Calculation of concentration
- Tube 7
- starting Fe3
- starting SCN-
- Fe(SCN)2 Absorption/slope
- Equilibrium Fe3 Fe3i - Fe(SCN)2e
- Equilibrium SCN- SCN-i - Fe(SCN)2e
- Equilibrium constant K Fe(SCN)2e / Fe3e
SCN-e
17
- Next Week Oct 29
- Exp 14B Full lab report including graph for all
the results - Exp 15 The Relative Strength of Some Acids
- Lab preparations
- Read background and procedure
- Protocol
- Chemicals HCl, H3PO4, NaH2PO4, CH3COOH, NH4NO3 ,
Al(NO3)3 , Zn(NO3)2 - Prelab assignment