Chapter 9: Chemical Equilibrium - PowerPoint PPT Presentation
Title:
Chapter 9: Chemical Equilibrium
Description:
Chapter 9: Chemical Equilibrium The forward and reverse reaction are both taking place at the same rate – PowerPoint PPT presentation
Number of Views:246
Avg rating:3.0/5.0
Title: Chapter 9: Chemical Equilibrium
1
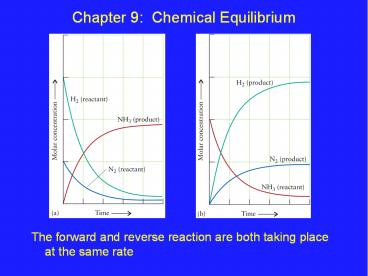
Chapter 9 Chemical Equilibrium
- The forward and reverse reaction are both taking
place at the same rate
2
Production and Decomposition of Ammonia
Forward Reaction N2 (g) 3H2 (g) ? 2NH3 (g)
Reverse Reaction 2NH3 (g) ? N2 (g) 3H2 (g)
Equilibrium Reaction N2 (g) 3H2 (g) ? 2NH3 (g)
Note the double headed arrow!
The ammonia is decomposing as fast as it is being
made at equilibrium
3
Equilibrium and the Law of Mass Action
- 2SO2 (g) O2 (g) ? 2SO3 (g)
5 mixtures of different initial compositions of
gases were made and allowed to reach equilibrium
at 1000K At first, you dont see a trend in the
data
4
Equilibrium and the Law of Mass Action
No trends, but if you calculate
You get the same value, regardless of initial
concentration Note K is unitless!
5
The Equilibrium Constant
K is the equilibrium constant for the reaction
6
The Equilibrium Constant
At equilibrium, the composition of the reaction
mixture can be expressed in terms of an
equilibrium constant where
For ideal gases, the concentrations are the
partial pressures of the individual gases For
solutions, the concentrations are the molar
values of the individual atoms/ions/molecules
7
Examples of K setup
aA (g) bB (g) ? cC (g) dD (g)
?
8
Units and Equilibrium Constants
- When working with equilibrium Constants, well
use the following unit conventions - Gases Units are bar
- Aqueous Solutions Unit is Molarity
- Solids The number 1
Solids have a single value (1) because the
concentration of a solid doesnt change.
9
Thermodynamic Origin of Equilibrium Constants
- The Free Energy changes as the composition of the
reaction mixture changes - All reactions will proceed towards equilibrium
(by either forward or reverse reaction) - ?Gº is the free energy difference b/w the pure
products and pure reactants
10
Thermodynamic Origins of Equilibrium Constants
- We can calculate the Free Energy change at any
point along the reaction coordinate with the
equation
aA (g) bB (g) ? cC (g) dD (g)
?Gr is the textbook Free Energy of reaction ?Gr
is the Free Energy of value when the reactants
and products are at particular concentrations
?
11
Example
- The standard free energy of reaction for
- 2SO2 (g) O2 (g) ? 2SO3 (g)
- Is ?Gr -141.74 kJ/mole at 25C. What is the
Gibbs Free Energy of reaction when the partial
pressure of each gas is 100.0 bar?
12
Example
- The Standard Gibbs Free Energy of Reaction for
- N2O4 (g) --gt 2NO2 (g)
- Is ?Gr 4.73 kJ/mole at 298K. What is the
value of ?Gr when the partial pressures are PN2O4
0.8 bar and PNO2 2.10 bar?
13
Free Energy of a Reaction at Equilibrium
- QK at equilibrium
- At equilibrium, ?G___
- Therefore,
- ?G ?Grº RTlnK
- ?Grº -RTlnK (only at equilibrium)
- We can use this to compute equilibrium constants
from ?Grº values
14
K and the Extent of Reactions
- When K is very large, the reaction favors the
products - When K is very small, the reaction favors the
reactants - When K1, the reaction is neither reactant nor
product favored (Equilibrium)
15
The Direction of Reaction
- How can we tell if a reaction will continue
towards the products or back towards the
reactants at a given point along the reaction
coordinate?
Q Reaction quotient used at any point in the
coordinate K Equilibrium constant
When QltK, ?G is negative (product favored) When
QK, ?G 0 When QgtK, ?G is positive (reactant
favored)
16
Equilibrium Calculations
Toolbox 9.1 Know it. Love it. Use it.
?
17
Example
- Under certain conditions, nitrogen and oxygen
react to form dinitrogen oxide, N2O. Suppose
that 0.482 moles of N2 and 0.933 moles of O2 are
transferred to a reaction vessel of volume 10.0L
and allowed to form N2O _at_ 800K. At this
temperature, K3.2x10-28 for the reaction - 2N2 (g) O2 (g) ? 2N2O (g)
- What are the partial pressures of the gases at
equilibrium?
18
Example
- Chlorine and fluorine react at 2500K to produce
ClF and reach the equilibrium - Cl2 F2 ? 2ClF
- With an equilibrium constant value of 20. If a
gaseous mixture of 0.2 bar Cl2, 0.1 bar F2 and
0.1 bar ClF is allowed to reach equilibrium, what
is the partial pressure of ClF in the mixture?
19
LeChateliers Principle
When the equilibrium composition is perturbed by
adding or removing a reactant of product, the
reaction tends to proceed in the direction that
brings Q closer to that of K.
?
20
Consider the Equilibrium Reaction
4NH3 (g) 3O2 (g) ? 2N2 (g) 6H2O (g)
- What would result from the
- Addition of N2
- Removal of NH3
- Removal of H2O
21
Effects of the Environment on Equilibria
- Compressing a Gas Phase Reaction
- The reaction shifts so as to decrease the
pressure - Decrease the number of gas molecules
- Changing the Temperature of a Reaction
- For exothermic reactions, lowering the
temperature causes a shift towards the products - For endothermic reactions, increasing the
temperature causes a shift towards the products