Chapter 2: The Chemistry of Life - PowerPoint PPT Presentation
1 / 21
Title:
Chapter 2: The Chemistry of Life
Description:
Chapter 2: The Chemistry of Life 2.1 Nature of Matter: atom: smallest part/basic unit of matter 3 sub-atomic particles: Proton (+), Neutron (0 ... – PowerPoint PPT presentation
Number of Views:192
Avg rating:3.0/5.0
Title: Chapter 2: The Chemistry of Life
1
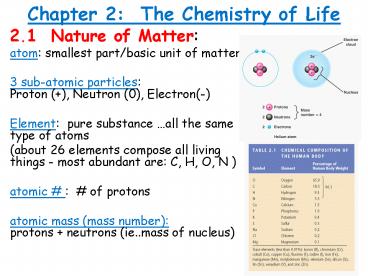
Chapter 2 The Chemistry of Life
- 2.1 Nature of Matter
- atom smallest part/basic unit of matter
- 3 sub-atomic particles
Proton (), Neutron (0), Electron(-) - Element pure substance all the same type of
atoms - (about 26 elements compose all living things -
most abundant are C, H, O, N ) - atomic of protons
- atomic mass (mass number) protons
neutrons (ie..mass of nucleus)
2
What are the element symbols for
- Carbon
- Hydrogen
- Oxygen
- Nitrogen
- Sulfur
- Phosphorus
- Sodium
- Chlorine
- Potassium
- Calcium
- Manganese
- Flourine
- Helium
- Magnesium
- Aluminum
- Iodine
- Lead
- Silver
- Lithium
- Mercury
3
What are the element symbols for
- Carbon C
- Hydrogen H
- Oxygen O
- Nitrogen N
- Sulfur S
- Phosphorus P
- Sodium Na
- Chlorine Cl
- Potassium K
- Calcium Ca
- Manganese Mn
- Flourine Fl
- Helium He
- Magnesium Mg
- Aluminum Al
- Iodine I
- Lead Pb
- Silver Ag
- Lithium Li
- Mercury Hg
4
2.1 Nature of Matter continued
- Isotopes elements with different neutron than
a stable atom - ex 14C, 3H, 32P, 34S
- Radioactive Isotopes have unstable nuclei
break down at a known rate, give off radioactive
particles (gamma rays, etc) - Dangerous AND useful, too
- ex fossil dating, bone scans, GI series,
chemotherapy - Compound substance formed by 2 or more elements
in a fixed ratio - physical and chemical properties of compound are
different than atoms composing the
compound - Molecule smallest unit of most compounds
5
BONDING PATTERNS
0
- 1. Ionic bonds attractions between ions of
opposite charge - when atoms gain or lose electrons, ions are
created
- 2. Covalent bonds join atoms into molecules
through electron sharing - when two atoms share one or more pairs of
outer shell electrons
CH4 ?
6
Polar Covalent/Non-polar Covalent/ H Bonds
0
- Non-polar Covalent When covalently bonded atoms
share electrons equally - Ex CO2
- Polar Covalent Electrons are shared unequally
between atoms, creating a polar molecule - Ex H20
- Hydrogen Bonds weak bonds important in the
chemistry of life - charged regions on water molecules are attracted
to the oppositely charged regions on nearby
molecules - Ex water to water (cohesion)
7
Why Water Supports All Life
0
- 1. Cohesion (ww) and Adhesion (wother)
- allows water to move from roots?leaves
- some insects can walk on water due to cohesive
surface tension - universal solvent can dissolve more solutes
than any other solvent - 2. Moderates temperature (heat capacity)
- takes a lot of energy to disrupt hydrogen bonds ?
water can absorb lots of heat without a large
rise in temp - As water cools? a slight drop in temp releases a
large amount of heat - water molecules take energy with it when it
evaporates ? evaporative cooling
8
Mixtures Solutions and Suspensions
- Mixture composed of 2 or more elements or
- compounds physically mixed, not chemically
combined - (ex salt and sugar together)
- 2 types of Mixtures
- a) Solution where components are evenly
distributed (ex salt water) - water solvent NaCl solute
- polarity of water allows it to dissolve ionic
compounds and polar molecules (ex salts, sugars,
minerals, gases, other solvents like alcohol) - b) Suspension when materials dont dissolve
in water, but break up into tiny pieces which do
not settle out (they are suspended by the moving
water) - ex blood (water has dissolved compounds,
blood cells and other components (lipids) which
remain suspended in mixture)
9
The chemistry of life is sensitive to acidic and
basic conditions
0
- Acid a compound that forms H ions in solution
- Base a compound that produces OH- (hydroxide)
ions in solution - Acidity or Alkalinity (base) is measured on
- the pH scale
- From 0 (most acidic) to 14 (most basic)
- The pH of most cells is kept close to 7 (neutral)
by buffers (substances that resist pH change) - Each step on pH scale is a factor of 10.
- (ex pH 5 is 10x more acidic than?)
Buffer weak acid or weak base which can keep a
pH stable ex Bicarbonate most important
buffer in body- maintains homeostasis in blood
When the number of H is equal to
the number of
OH- ? water H OH- ? H20
10
2.3 Organic Chemistry The Chemistry of Carbon
- Organic must contain at least one carbon.
CH4 simplest organic molecule - Carbon has 4 valence electrons
- Therefore, carbon will always make 4 bonds with
other atoms - Carbon can bond with other carbons, form chains,
rings - Ability to form millions of different compounds
with other elements
11
The Four Macromolecules of Life
- Macromolecule (polymer) made by joining many
monomers (single unit) - Polymerization chemical rxn which joins
monomers to make polymers
The four main classes of biological molecules 1.
Carbohydrates (sugar, starches, cellulose) 2.
Lipids (wax, fats, oils, steroids) 3. Proteins
(muscle, hair, hormones, enzymes) 4. Nucleic
acids (DNA and RNA)
12
1. CARBOHYDRATES Monomer Monosaccharide
- Contain C, H, and O in a 121 ratio
- Most end with ose
- An animals main energy source
- Carbs are burned first in the body
- Monosaccharides (C6H12O6)
- glucose, fructose, galactose
- Disaccharides (C12H22O11)
- sucrose, lactose, maltose
- Polysaccharides (complex carbohydrates)
- A) glycogen (carb storage animal liver)
- B) starch (carb storage in plants)
- C) cellulose (cell walls, cotton) roughage
- D) chitin (exoskeletons of arthropods)
13
2. LIPIDS Monomer Fatty Acids
- Mostly C and H atoms linked by
- nonpolar covalent bonds
- Reserve energy-storage molecules
- (burned after carbs are gone)
- Insoluble in water (polar)
- Soluble in nonpolar solvents (ether)
- More energy in lipids than in carbs
- - 9 cal/g Lipid vs. 4 cal/g Carb
- Examples triglycerides, phospholipids,
- steroids (cholesterol), waxes, oils, fats
- Triglyceride 3 fatty acids 1 glycerol
- Saturated Fats all single bonds in chain
- - solid at room temp (ex butter, lard)
- Unsaturated fats one or more CC bond in
chain - - liquid at room temp (ex all oils)
14
3. NUCLEIC ACIDS Monomer Nucleotide
- Nucleic acids (DNA and RNA) store and transmit
genetic information - DNA Deoxyribonucleic acid
- RNA Ribonucleic acid
- Large macromolecules containing C, H, O, N, P
- One nucleotide 5-carbon sugar, phosphate
(PO4-), nitrogenous base
The sugars and phosphates are the backbone for
the nucleic acid
DNAs sugar deoxyribose RNAs sugar ribose
15
4. PROTEINS Monomer Amino Acid
- Essential to the structures and
activities of life - Contain C, H, O, N (S, P)
- 50 of your dry weight
- examples of groups of proteins
- 1. enzymes (amylase, sucrase, maltase, lactase)
ase ending - 2. structural (collagen, elastin)
- 3. contractile (actin, myosin)
- 4. transport (hemoglobin, protein channels)
- 5. hormones (insulin)
16
AMINO ACID Structure
0
- Each amino acid has
- An amino group (-NH2)
- A carboxyl group (COOH)
- An R group, which distinguishes each of the 20
different amino acids
- Each amino acid has specific properties based
on the R-group - Peptide bonds link amino acids together ?
polypeptide (protein)
17
PROTEINS 4 Levels of Organization
- Amino acids are assembled into polypeptide chains
according to - instructions coded in the DNA.
Primary Structure the sequence of amino acids in
its polypeptide chain
Secondary structure the coiling or folding of
the chain
Tertiary Structure the overall
three-dimensional shape of a polypeptide
created when R-groups bond
Quaternary Structure the association of two or
more polypeptide chains
18
2.4 Chemical Reactions and Enzymes
- Chemical reaction process that changes or
transforms one set of chemicals into another - Those chemicals that enter into a reaction are
the reactants, those that are made are the
products - Chemical reactions change the bonding patterns in
the reactants - Energy is released or absorbed when chemical
bonds are formed or broken during a reaction - Rxns releasing energy generally happen
spontaneously - Rxns which absorb energy need energy to start
them - Some energy releasing rxns need activation (input
of )energy to get started
19
Enzymes are vital proteins that run biochemical
rxns
- Lower the activation energy (EA) of chemical
reactions (they are catalysts) - The reactants they work on are called
substrates - Most enzymes are named for their substrates
with an -ase ending - Ex sucrase digests sucrose lactase
digests lactose - VERY shape specific (lock and key) reaction
with active site on enzyme (where substrate and
enzyme join)
- One Enzyme One Substrate
- Enzymes have unique three-dimensional shapes so
they can fit onto their specific substrate - Shapes determine function and which chemical
reactions they can perform - All related to their 3-D folding pattern born
from?
20
Factors Which Affect Enzyme Activity
- Temperature, salt concentration, pH,
inhibitors - Enzyme inhibitors can alter enzyme function
- Competitive inhibitor blocks active site,
substrate cant attach and remains unchanged - Non-competitive inhibitor alters enzymes
function by changing its shape - Many poisons, pesticides, and drugs are enzyme
inhibitors
- Some food for thought
- Why do we put lemon juice on apples?
- What is the purpose of a fever?
- What happens when a raw egg hits a hot fry pan?
- Why do we put produce/perishables in the fridge?
- How does a Siamese cat get its color pattern?
21
GENES Sequences of DNA
0
- DNA sequences spell out the amino acid sequences
of proteins - Mutations in the DNA sequence ? wrong amino acid
sequence ? wrong protein shape ? no function - Ex Lactose Intolerance
- Mutations in lactase gene?
mutations in lactase amino acid chain
sequence ? defective lactase shape?
enzyme cant
fit onto lactose substrate ?
lactose does not get digested. - Q Why is it a big deal?
- A If YOU dont digest the lactose in your
digestive tract, all the E.coli willall of their
waste made from eating all this food will leave
you with cramps, bloating, and diarrheanot fun!
Lactase enzyme