Synthesis Reactions - PowerPoint PPT Presentation
1 / 12
Title:
Synthesis Reactions
Description:
Synthesis Reactions Also known as direct combination reactions. Two or more reactants form a single product. Generic Example: A + B AB Synthesis Reactions Synthesis ... – PowerPoint PPT presentation
Number of Views:26
Avg rating:3.0/5.0
Title: Synthesis Reactions
1
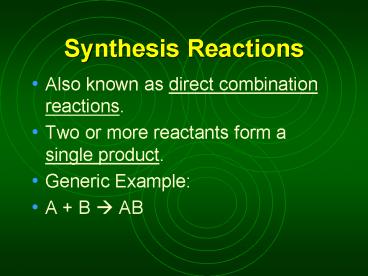
Synthesis Reactions
- Also known as direct combination reactions.
- Two or more reactants form a single product.
- Generic Example
- A B ? AB
2
Synthesis Reactions
K1 and Cl-1
Example K Cl2 ?
KCl
2
2
Remember to Balance!
3
Synthesis Reactions
Al3 and Br-1
Example Al Br2 ?
AlBr3
3
2
2
Remember to Balance!
4
Decomposition Reactions
- A single reactant is broken down into two or more
products. - Generic Example
- AB ? A B
5
Decomposition Reactions
Diatomic!!!
2
Example NaCl ?
Cl2
2
Na
Remember to Balance!
6
Decomposition Reactions
Diatomic!!!
O2
Example Al2O3 ?
3
2
Al
4
Remember to Balance!
7
Single-Replacement Rxns
- A single element replaces an element in a
compound to form a new compound and element. - Generic Example
- A BX ? AX B
Click here for a video that shows Ag replacing Cu
8
Single-Replacement Rxns
- You must use the Activity Series to determine if
a reaction will work! - Metals will replace other metals that appear
below them - (Pg. 295)
http//cwx.prenhall.com/bookbind/pubbooks/hillche
m3/medialib/media_portfolio/text_images/CH04/FG04_
15.JPG
9
Single-Replacement Rxns
Mg2 and NO3-1
Mg(NO3)2
Ag
2
Mg AgNO3?
2
Replaced Metal
Mg is above Ag, so the reaction will work!
http//cwx.prenhall.com/bookbind/pubbooks/hillche
m3/medialib/media_portfolio/text_images/CH04/FG04_
15.JPG
10
Single-Replacement Rxns
No Reaction
NR
Cu FeSO4?
Cu is below Fe, so the reaction will NOT work!
http//cwx.prenhall.com/bookbind/pubbooks/hillche
m3/medialib/media_portfolio/text_images/CH04/FG04_
15.JPG
11
Single-Replacement Rxns
- Non-metals will replace other non-metals that are
below them on the periodic table.
http//www.avon-chemistry.com/ch9_chart11.jpg
12
Single-Replacement Rxns
K1 and Cl-1
KCl
I2
2
Cl2 KI?
2
Diatomic!
Cl is above I, so the reaction will work!
http//www.avon-chemistry.com/ch9_chart11.jpg